Is Air A Mixture Or A Compound
News Leon
Mar 28, 2025 · 5 min read
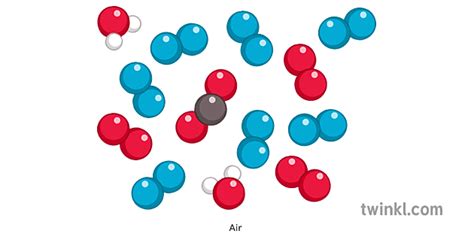
Table of Contents
Is Air a Mixture or a Compound? A Deep Dive into Atmospheric Composition
The question of whether air is a mixture or a compound is a fundamental one in chemistry and atmospheric science. While seemingly simple, the answer requires a nuanced understanding of the definitions of mixtures and compounds, and the specific composition of air itself. This article will delve deep into the chemical nature of air, exploring its components and the properties that definitively classify it as a mixture, not a compound.
Understanding the Difference: Mixtures vs. Compounds
Before we analyze air, let's clarify the key distinction between mixtures and compounds. This distinction is crucial for correctly classifying any substance, including air.
Compounds: Chemically Bonded
A compound is a substance formed when two or more chemical elements are chemically bonded together. This bonding involves the sharing or transfer of electrons, resulting in a new substance with entirely different properties from its constituent elements. For example, water (H₂O) is a compound formed from the chemical bonding of hydrogen and oxygen. The properties of water are distinctly different from those of hydrogen and oxygen in their pure forms. The composition of a compound is fixed and represented by a chemical formula (e.g., H₂O, NaCl). Breaking down a compound requires a chemical reaction.
Mixtures: Physically Combined
A mixture, on the other hand, is a substance composed of two or more components that are physically combined but not chemically bonded. The components retain their individual properties, and their proportions can vary. For example, a saltwater solution is a mixture of salt and water. The salt dissolves in the water, but the individual components remain distinct; they can be separated through physical methods like evaporation.
The Composition of Air: A Detailed Look
Air, the gaseous mixture that surrounds our planet, is primarily composed of:
-
Nitrogen (N₂): Approximately 78% of air is nitrogen, a relatively inert diatomic gas. Its presence is crucial for maintaining the balance of atmospheric gases and supporting life.
-
Oxygen (O₂): Making up about 21% of air, oxygen is vital for respiration in most living organisms. It is a highly reactive gas, essential for combustion and various metabolic processes.
-
Argon (Ar): Present in roughly 0.93%, argon is a noble gas, meaning it's chemically unreactive. It plays a minimal role in most biological processes but contributes to the overall atmospheric composition.
-
Other Gases: In addition to these major components, air contains trace amounts of other gases, including:
-
Carbon dioxide (CO₂): While present in a small percentage (around 0.04%), CO₂ plays a critical role in the Earth's climate and is essential for photosynthesis. Its levels are increasing due to human activities, contributing to global warming.
-
Neon (Ne), Helium (He), Methane (CH₄), Krypton (Kr), Hydrogen (H₂), Nitrous Oxide (N₂O), Xenon (Xe), Ozone (O₃): These gases are present in even smaller concentrations and have various roles in atmospheric chemistry and climate processes.
-
Why Air is a Mixture: Evidence and Arguments
Several key characteristics of air solidify its classification as a mixture:
-
Variable Composition: The proportions of gases in air can vary slightly depending on location, altitude, and other factors. For example, air at higher altitudes contains less oxygen and more nitrogen. This variable composition is a hallmark of mixtures, not compounds. A compound always has a fixed composition.
-
Retention of Individual Properties: Each gas in air retains its individual chemical properties. Nitrogen remains inert, oxygen remains reactive, and so forth. If air were a compound, the properties of its constituent elements would be fundamentally altered upon bonding.
-
Separation by Physical Means: The components of air can be separated using physical methods, such as fractional distillation of liquid air. This process utilizes differences in boiling points to isolate the individual gases. The ability to separate components through physical means without chemical reactions is a defining characteristic of mixtures.
-
No Chemical Bonds: The gases in air are not chemically bonded to one another. They are simply intermingled, held together by weak intermolecular forces. The absence of chemical bonds is definitive evidence that air is a mixture.
-
No Fixed Ratio: The ratio of gases in air is not fixed by a chemical formula. This contrasts sharply with compounds, which always have a specific and unchanging ratio of elements, as dictated by their chemical formula.
The Importance of Understanding Air's Nature
Knowing that air is a mixture, not a compound, has significant implications across numerous scientific disciplines:
-
Atmospheric Science: Understanding the composition and variability of air is crucial for climate modeling, weather forecasting, and studying atmospheric pollution.
-
Environmental Science: The knowledge that air is a mixture allows us to monitor and manage the levels of various gases, including pollutants, that impact air quality and human health.
-
Biology: The specific gases in air, especially oxygen and carbon dioxide, play vital roles in respiration and photosynthesis, which are fundamental to life on Earth.
-
Chemistry: Studying the behavior of individual gases in air helps us understand reaction kinetics, equilibrium, and various chemical processes occurring in the atmosphere.
-
Engineering: The properties of air, being a mixture, are important considerations in various engineering applications, such as aerospace engineering, combustion engineering, and HVAC systems.
Addressing Common Misconceptions
Some individuals might mistakenly believe that air is a compound due to the presence of certain molecules like oxygen (O₂) and nitrogen (N₂). However, these are diatomic molecules—molecules composed of two atoms of the same element—not compounds formed from different elements. The crucial factor is the lack of chemical bonding between the different gases present in air.
Conclusion: Air as a Homogeneous Mixture
In conclusion, overwhelming evidence supports the classification of air as a homogeneous mixture of gases. Its variable composition, the retention of individual gas properties, the separation by physical means, the absence of chemical bonds, and the lack of a fixed ratio all point definitively to its mixture status. Understanding the nature of air as a mixture is essential for advancing scientific knowledge across various fields and addressing critical environmental challenges. The seemingly simple question of "Is air a mixture or a compound?" underscores the importance of a precise understanding of fundamental scientific concepts.
Latest Posts
Latest Posts
-
Who Is The Father Of Bio
Mar 31, 2025
-
Which Of The Following Is True About Genes
Mar 31, 2025
-
What Animal Has The Largest Breasts
Mar 31, 2025
-
Which Two Bonds Are Most Similar In Polarity
Mar 31, 2025
-
13 With 3 Repeating As A Fraction
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Air A Mixture Or A Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
