Which Atom Contains Exactly 15 Protons
News Leon
Mar 23, 2025 · 5 min read
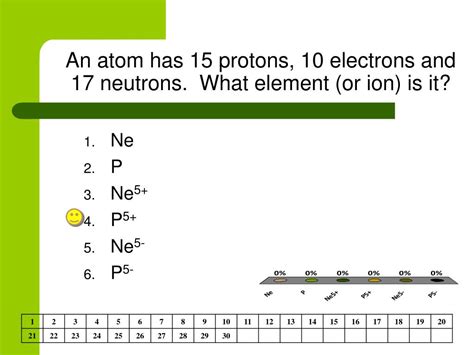
Table of Contents
Which Atom Contains Exactly 15 Protons? Unlocking the Secrets of Phosphorus
The question, "Which atom contains exactly 15 protons?" leads us on a fascinating journey into the heart of atomic structure and the periodic table of elements. The answer, simply put, is phosphorus (P). But understanding why this is the case requires delving into the fundamental concepts of atomic number, isotopes, and the significance of protons in defining an element's identity.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the element with 15 protons, let's establish a solid foundation in atomic structure. Every atom is composed of three subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons determines the element's atomic number and its unique identity.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons generally equals the number of protons in a neutral atom.
The atomic number is the defining characteristic of an element. It's the number of protons in the nucleus of an atom. This number is unique to each element and is crucial in organizing the periodic table. Elements are arranged in increasing order of their atomic numbers.
The Significance of the Atomic Number: 15 and Phosphorus
The question itself gives us the key: 15 protons. This directly translates to the atomic number. Consulting the periodic table, we find that the element with atomic number 15 is phosphorus (P). Therefore, any atom containing precisely 15 protons is an atom of phosphorus.
Isotopes: Variations in Neutron Number
While the number of protons defines the element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. For instance, phosphorus has several isotopes, including:
- Phosphorus-31 (³¹P): This is the most common and stable isotope of phosphorus. It contains 15 protons and 16 neutrons (15 + 16 = 31).
- Phosphorus-32 (³²P): This is a radioactive isotope of phosphorus, containing 15 protons and 17 neutrons. It's used in various applications, including medical imaging and biological research.
- Other isotopes: Several other phosphorus isotopes exist, but they are all radioactive and have shorter half-lives.
The notation ³¹P (or ³²P, etc.) indicates the mass number, which is the total number of protons and neutrons in the nucleus. The superscript number represents the mass number, while the symbol 'P' denotes phosphorus. It's vital to remember that although the neutron number can vary, the proton number (atomic number) always remains 15 for all phosphorus isotopes.
The Properties and Importance of Phosphorus
Phosphorus, the element with 15 protons, plays a crucial role in various biological and industrial processes. Its importance stems from its unique chemical properties:
- Essential Nutrient: Phosphorus is an essential element for all living organisms. It's a crucial component of DNA and RNA, the molecules that carry genetic information. It's also vital for energy transfer in cells (ATP) and the structure of bones and teeth.
- Fertilizers: Phosphorus compounds are extensively used in fertilizers to enhance plant growth and increase crop yields. This is because phosphorus is a vital nutrient for plant development.
- Industrial Applications: Phosphorus is also used in various industrial applications, including the production of detergents, matches, and flame retardants. Its reactivity and ability to form strong bonds contribute to its diverse uses.
Exploring the Periodic Table: Neighboring Elements and Trends
Understanding phosphorus within the context of the periodic table enhances our comprehension of its properties. Phosphorus is located in Group 15 (also known as the pnictogens) and Period 3. Its neighbors on the periodic table help illustrate periodic trends:
- Nitrogen (N): Located above phosphorus in Group 15, nitrogen has one less proton (7 protons). Both elements share similarities in their chemical behavior, although phosphorus shows some distinct differences due to its larger size and increased number of electron shells.
- Sulfur (S): Situated to the right of phosphorus, sulfur (16 protons) displays different chemical properties due to its position in a different group. This highlights how the arrangement of electrons, influenced by the number of protons, dramatically impacts an element's behavior.
- Silicon (Si): Located to the left of phosphorus in Period 3, silicon (14 protons) demonstrates contrasting characteristics, highlighting the influence of group differences.
Delving Deeper: Nuclear Chemistry and Radioactive Isotopes
The existence of radioactive phosphorus isotopes, such as ³²P, opens the door to understanding nuclear chemistry. Radioactive isotopes decay over time, emitting particles and energy. This decay process is governed by the isotope's half-life – the time it takes for half of the radioactive atoms to decay.
³²P, with its relatively short half-life, finds applications in scientific research. It can be incorporated into biological molecules to track their movement and behavior within cells. This technique is widely used in medical research and diagnostics.
Conclusion: The Uniqueness of Phosphorus and Atomic Number
In conclusion, the element containing exactly 15 protons is phosphorus (P). This seemingly simple fact highlights the fundamental importance of the atomic number in defining an element's identity and properties. Understanding atomic structure, isotopes, and the periodic table allows us to appreciate the unique role phosphorus plays in various biological and industrial processes. The use of radioactive isotopes like ³²P further illustrates the sophisticated applications stemming from our understanding of atomic structure. The seemingly simple question – "Which atom contains exactly 15 protons?" – ultimately unveils a rich tapestry of scientific knowledge. This exploration underscores the elegance and precision of the laws governing the atomic world. Further exploration of the periodic table, atomic structure, and nuclear chemistry will reveal even more fascinating details regarding the behaviour of atoms and their unique properties. The inherent beauty of science lies in the ability to start with a basic question and uncover a universe of knowledge and application.
Latest Posts
Latest Posts
-
How Many Neutrons Does Uranium 238 Have
Mar 24, 2025
-
How Many Lone Pairs Does Chlorine Have
Mar 24, 2025
-
Water At Room Temperature Is A Liquid
Mar 24, 2025
-
In What Organelle Does Cellular Respiration Take Place
Mar 24, 2025
-
How Is Your Little Fish And Cute Hamster
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Which Atom Contains Exactly 15 Protons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
