When Ice Melts Does The Volume Change
News Leon
Mar 31, 2025 · 5 min read
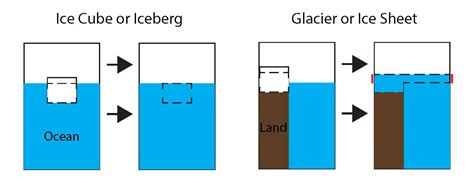
Table of Contents
When Ice Melts, Does the Volume Change? Exploring the Density Anomaly of Water
Water, the elixir of life, is a substance so ubiquitous that we often take its remarkable properties for granted. One of these fascinating characteristics is its anomalous behavior upon freezing and melting. Unlike most substances, water expands when it freezes, forming ice that is less dense than its liquid counterpart. But what happens to the volume when ice melts? This seemingly simple question delves into the intricate world of molecular structure, density, and the crucial role water plays in our environment.
The Density Difference: Why Ice Floats
The key to understanding the volume change lies in the unique molecular structure of water. Water molecules (H₂O) are polar, meaning they possess a slight positive charge on the hydrogen atoms and a slight negative charge on the oxygen atom. This polarity leads to strong hydrogen bonds between molecules.
Hydrogen Bonding: The Architect of Ice's Structure
In liquid water, these hydrogen bonds are constantly forming and breaking, creating a dynamic and relatively disordered structure. However, when water freezes, the molecules arrange themselves into a highly ordered crystalline lattice. This lattice structure, characterized by hexagonal rings of water molecules, is significantly more spacious than the more disordered arrangement in liquid water.
This increased spacing is the primary reason why ice is less dense than liquid water. The same number of molecules occupies a larger volume in the solid (ice) phase than in the liquid phase. This lower density is why ice floats – a phenomenon vital for aquatic life and the Earth's climate.
The Volume Change During Melting: A Decrease in Volume
Now, let's address the central question: when ice melts, does the volume change? Yes, the volume decreases. As the ice melts, the rigid crystalline structure breaks down. The water molecules gain more freedom of movement, and the average distance between them decreases. They pack more closely together, resulting in a reduction in overall volume.
This volume decrease might seem counterintuitive, given that ice is less dense than water. However, remember that density is mass per unit volume. While the mass remains constant during the phase transition, the volume decreases, causing the density to increase.
Quantifying the Volume Change: Understanding the Percentage Difference
The exact percentage decrease in volume when ice melts depends on several factors, including temperature and pressure. However, a reasonable approximation is that ice melts to roughly 92% of its initial volume. This means that a block of ice with a volume of 100 cubic centimeters will reduce to approximately 92 cubic centimeters upon complete melting.
This seemingly small decrease in volume can have significant consequences on a larger scale, especially in natural processes such as river ice breakup and glacial melt.
The Implications of the Volume Change: Real-World Applications and Environmental Significance
The density anomaly of water and the consequent volume change during melting have far-reaching implications for various aspects of our world:
1. Aquatic Ecosystems: Life Under the Ice
The fact that ice floats is crucial for the survival of aquatic life. A layer of ice on the surface of a lake or ocean acts as an insulator, preventing the water underneath from freezing completely. This allows aquatic organisms to survive even in sub-zero temperatures. If ice were denser than water, it would sink to the bottom, leading to the potential freezing of entire bodies of water, with devastating effects on aquatic life.
2. Weather Patterns and Climate Regulation: A Global Impact
The density anomaly of water influences global weather patterns and climate regulation. The melting of ice, particularly polar ice caps and glaciers, contributes to sea-level rise. This volume change, even though a seemingly small percentage for individual ice blocks, adds up when scaled to the vast quantities of ice found on our planet.
3. Engineering and Construction: Considerations for Ice Formation
Engineers and construction professionals must account for the volume changes associated with ice formation and melting in their designs. Pipes and other structures must be able to withstand the expansion of freezing water, while the volume decrease during melting might impact structural stability in some cases. This is particularly crucial in cold climates.
4. Geological Processes: Shaping Landscapes Through Freeze-Thaw Cycles
Freeze-thaw cycles, the repeated freezing and melting of water, contribute significantly to geological processes, particularly weathering and erosion. The expansion of water as it freezes can fracture rocks and contribute to the formation of soil. The subsequent melting and reduction in volume can further influence the movement and redistribution of sediment.
Beyond the Basics: Factors Affecting the Volume Change
While the basic principle of volume decrease during ice melting is well-established, several factors can influence the precise magnitude of this change:
1. Temperature: The Rate of Melting
The temperature at which melting occurs affects the rate of volume change. Faster melting might lead to slight variations in the final volume compared to slower melting.
2. Pressure: Compressing the Ice
Pressure influences the melting point of ice. Increased pressure lowers the melting point, potentially affecting the volume change during the phase transition.
3. Impurities: The Presence of Dissolved Substances
The presence of dissolved substances in the water can affect the density of both the ice and the liquid water, leading to subtle variations in the volume change during melting.
Conclusion: A Phenomenon with Profound Implications
The seemingly simple question of whether the volume changes when ice melts reveals a complex interplay of molecular forces, phase transitions, and macroscopic consequences. The unique density anomaly of water, leading to a decrease in volume upon melting, has profound implications for aquatic ecosystems, climate regulation, engineering, geological processes, and more. Understanding this fundamental property of water is essential for comprehending many natural phenomena and for developing sustainable solutions to environmental challenges. The expansion of water upon freezing and subsequent contraction upon melting highlight the fascinating and multifaceted nature of this essential substance. Further research continues to unravel the nuances of this unique property, expanding our understanding of water's pivotal role in our world.
Latest Posts
Latest Posts
-
A Process By Which Information Is Exchanged Between Individuals
Apr 02, 2025
-
Select The Four Statements About Plasmodium That Are True
Apr 02, 2025
-
Greatest Common Factor Of 36 And 20
Apr 02, 2025
-
What Is The Antonym Of Urban
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about When Ice Melts Does The Volume Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
