What Type Of Bond Is Mgo
News Leon
Mar 28, 2025 · 6 min read
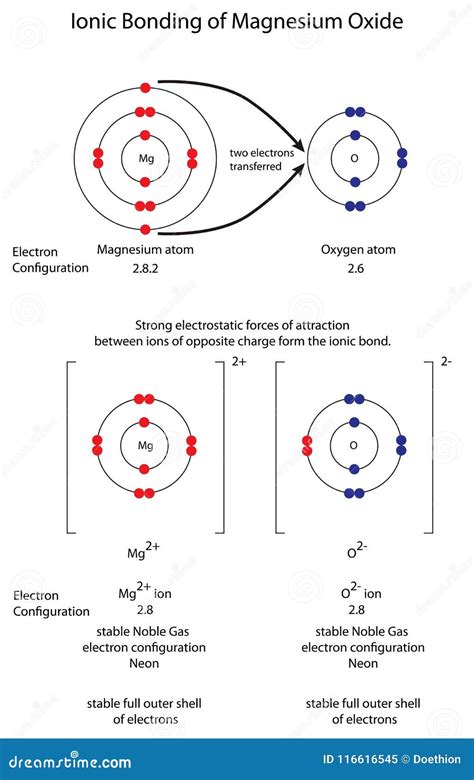
Table of Contents
What Type of Bond is MgO? Delving into the Ionic Bond of Magnesium Oxide
Magnesium oxide (MgO), also known as magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (Mg). Understanding the type of bond present in MgO is crucial to comprehending its properties and applications. This article delves deep into the nature of the bond in MgO, exploring its ionic character, the electronegativity difference between magnesium and oxygen, and the resulting properties of this important compound. We'll also examine related concepts like lattice energy and compare it to other types of chemical bonds.
The Ionic Bond: A Foundation of MgO's Structure
The dominant type of bond in MgO is an ionic bond. An ionic bond is formed through the electrostatic attraction between oppositely charged ions. This happens when one atom (typically a metal) loses one or more electrons to become a positively charged ion (cation), and another atom (usually a non-metal) gains these electrons to become a negatively charged ion (anion). In MgO, magnesium (Mg) loses two electrons to become a Mg²⁺ cation, and oxygen (O) gains these two electrons to become an O²⁻ anion. This transfer of electrons results in a strong electrostatic attraction between the positively charged magnesium ions and the negatively charged oxygen ions.
Electronegativity: The Driving Force Behind Ionic Bonds
The concept of electronegativity plays a crucial role in determining the type of bond formed between two atoms. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Oxygen has a significantly higher electronegativity than magnesium. This large electronegativity difference is the driving force behind the complete transfer of electrons from magnesium to oxygen, leading to the formation of the ionic bond in MgO.
Comparing Electronegativities: Mg vs. O
Oxygen has an electronegativity of approximately 3.5 on the Pauling scale, while magnesium has an electronegativity of around 1.2. The difference (3.5 - 1.2 = 2.3) is substantial, indicating a strong tendency for electron transfer and the formation of a predominantly ionic bond. This large difference signifies a significant ionic character in the MgO bond.
Lattice Structure and Properties Arising from Ionic Bonding
The ionic bonds in MgO arrange themselves in a highly ordered crystalline structure known as a rock salt crystal lattice or face-centered cubic (FCC) structure. In this structure, each Mg²⁺ ion is surrounded by six O²⁻ ions, and each O²⁻ ion is surrounded by six Mg²⁺ ions. This arrangement maximizes the electrostatic attraction between the oppositely charged ions, contributing to the high melting point and hardness of MgO.
Properties stemming from the Ionic Bond in MgO:
-
High Melting Point: The strong electrostatic forces between the Mg²⁺ and O²⁻ ions require a significant amount of energy to overcome, resulting in a high melting point (around 2852 °C). This high melting point indicates the strength of the ionic bond.
-
Hardness: The rigid, tightly packed crystal lattice structure contributes to MgO's hardness. The strong ionic bonds resist deformation.
-
Brittleness: Although hard, MgO is brittle. When stress is applied, the layers of ions can easily shift, disrupting the electrostatic attraction and causing the crystal to fracture along planes of weakness.
-
Insulator: In the solid state, the electrons are tightly bound within the ions, and there are no free electrons available for conduction. This makes MgO a good electrical insulator. However, molten MgO becomes a conductor.
-
High Heat Capacity: MgO's ability to absorb significant heat energy before undergoing a temperature change is directly related to the strong ionic interactions within its crystalline structure.
-
Solubility: MgO exhibits low solubility in water but readily reacts with acids. This behaviour is also characteristic of ionic compounds.
Comparison with Other Bond Types: Covalent and Metallic Bonds
It's important to understand how the ionic bond in MgO differs from other types of chemical bonds:
Covalent Bonds: Sharing Electrons
In a covalent bond, atoms share electrons to achieve a stable electron configuration. Unlike MgO, where electrons are completely transferred, covalent bonds involve the sharing of electron pairs. Molecules like water (H₂O) and methane (CH₄) are examples of compounds with primarily covalent bonds. The electronegativity difference between the atoms involved in a covalent bond is usually small.
Metallic Bonds: Electron Sea Model
Metallic bonds occur in metals where valence electrons are delocalized, forming a "sea" of electrons that are shared among all the metal atoms. This electron sea allows for good electrical and thermal conductivity. Metals like copper (Cu) and iron (Fe) exhibit metallic bonding.
Beyond the Ideal: Partial Ionic Character
While the MgO bond is predominantly ionic, it's not entirely purely ionic. A small degree of covalent character may exist due to the polarization of the ions. This polarization arises from the uneven distribution of electron density within the ions, resulting in a slight overlap of electron clouds. However, this covalent contribution is minor compared to the dominant ionic character.
Factors Influencing Ionic Character
The degree of ionic character in a bond can be influenced by several factors, including:
-
Electronegativity Difference: A larger electronegativity difference between atoms generally leads to greater ionic character.
-
Size of Ions: Smaller ions tend to have a higher charge density, increasing the electrostatic attraction and enhancing ionic character.
-
Polarizability: The ease with which an ion's electron cloud can be distorted affects the extent of covalent character.
Applications of MgO: Leveraging its Properties
The unique properties of MgO, arising from its ionic bonding, make it suitable for various applications:
-
Refractory Materials: MgO's high melting point makes it ideal for use in high-temperature applications, such as furnace linings and crucibles.
-
Cement and Construction: MgO is used as a component in cements and other construction materials.
-
Medical Applications: MgO is used as an antacid and laxative.
-
Agriculture: MgO is used as a soil amendment to correct magnesium deficiency.
-
Industrial Catalyst: Its ability to participate in chemical reactions makes it useful in certain catalytic applications.
Conclusion: The Significance of Ionic Bonding in MgO
The ionic bond is the fundamental type of bond present in magnesium oxide (MgO). The significant electronegativity difference between magnesium and oxygen drives the complete transfer of electrons, resulting in the formation of Mg²⁺ and O²⁻ ions and a strong electrostatic attraction between them. This ionic bonding gives rise to MgO's characteristic properties – high melting point, hardness, brittleness, insulating nature, and its wide range of industrial and medical applications. While a small degree of covalent character may exist, the ionic nature of the bond remains its dominant feature, making it a classic example of an ionic compound and a cornerstone of materials science and chemistry. Understanding the nature of the bond in MgO provides valuable insights into its behaviour and facilitates its effective use in various technological and industrial settings. Further research continues to explore the subtle nuances of its bonding and discover new potential applications for this versatile material.
Latest Posts
Latest Posts
-
Is Boiling Point Chemical Or Physical
Mar 31, 2025
-
Which Of The Following Is The Most Stable Cation
Mar 31, 2025
-
Who Is The Father Of Bio
Mar 31, 2025
-
Which Of The Following Is True About Genes
Mar 31, 2025
-
What Animal Has The Largest Breasts
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bond Is Mgo . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
