What Temperature Does Water Boil Kelvin
News Leon
Mar 18, 2025 · 5 min read
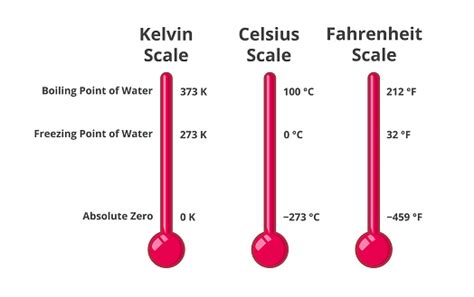
Table of Contents
What Temperature Does Water Boil in Kelvin? A Deep Dive into Boiling Points and Thermodynamics
The seemingly simple question, "What temperature does water boil in Kelvin?" opens a door to a fascinating exploration of thermodynamics, phase transitions, and the very nature of temperature scales. While a quick search might give you the answer, understanding why water boils at that specific Kelvin temperature requires delving into the underlying scientific principles. This article will not only provide the answer but also equip you with a comprehensive understanding of the concept.
Understanding Temperature Scales
Before diving into the boiling point of water in Kelvin, let's refresh our understanding of different temperature scales. We commonly use three scales:
- Celsius (°C): Based on the freezing (0°C) and boiling (100°C) points of water at standard atmospheric pressure.
- Fahrenheit (°F): Another widely used scale, with water freezing at 32°F and boiling at 212°F.
- Kelvin (K): The absolute temperature scale, where 0 K represents absolute zero – the theoretical point where all molecular motion ceases. There are no negative Kelvin temperatures.
The Kelvin scale is crucial in scientific applications because it directly relates to the kinetic energy of molecules. Higher Kelvin temperatures mean molecules possess more kinetic energy and move more vigorously.
The Boiling Point of Water: A Phase Transition
Water boils when its vapor pressure equals the surrounding atmospheric pressure. This is a phase transition, a change in the physical state of matter from liquid to gas. The energy required to overcome the intermolecular forces holding water molecules together in the liquid phase is provided by heat.
At sea level, under standard atmospheric pressure (1 atmosphere or 101.325 kPa), water boils at:
- 100°C
- 212°F
- 373.15 K
This is the answer to our initial question. Water boils at 373.15 Kelvin.
Factors Affecting the Boiling Point
While 373.15 K is the standard boiling point, several factors can influence the actual boiling temperature:
1. Atmospheric Pressure: The Altitude Effect
Atmospheric pressure decreases with altitude. At higher elevations, there are fewer air molecules pressing down on the water's surface. This means the water's vapor pressure needs to reach a lower value to equal the surrounding pressure, resulting in a lower boiling point. On top of Mount Everest, for example, water boils at a significantly lower temperature than at sea level.
2. Impurities in Water
Dissolved substances in water can elevate its boiling point. This phenomenon is known as boiling point elevation. The extent of the elevation depends on the concentration of the dissolved substances. Seawater, with its dissolved salts, boils at a slightly higher temperature than pure water.
3. Presence of Dissolved Gases
Dissolved gases in water can also affect the boiling point. These gases can form bubbles that interfere with the formation of vapor bubbles, potentially leading to slight variations in the boiling point.
4. Container Material and Shape
The material and shape of the container can influence heat transfer and nucleation (the formation of bubbles), which might slightly affect the observed boiling temperature. However, these effects are generally minor compared to the influence of pressure and impurities.
The Role of Kinetic Energy and Molecular Motion
The boiling point is intrinsically linked to the kinetic energy of water molecules. As heat is added to liquid water, the molecules absorb energy and move faster. When their kinetic energy surpasses the intermolecular forces holding them together in the liquid state, they overcome these forces and transition into the gaseous phase (steam). At 373.15 K, the average kinetic energy of water molecules is sufficient for this phase transition to occur under standard atmospheric pressure.
Applications of Understanding Boiling Point
Understanding the boiling point of water, and the factors that affect it, has numerous practical applications:
- Cooking: Altitude significantly impacts cooking times because water boils at a lower temperature at higher altitudes. Recipes might need adjustment at higher elevations to account for this.
- Industrial Processes: Many industrial processes involve boiling or evaporating water. Understanding boiling point variations is crucial for optimizing these processes.
- Steam Generation: Steam power plants rely on the boiling of water to generate steam that drives turbines. Precise control of the boiling point is essential for efficient power generation.
- Scientific Experiments: Precise temperature control is vital in many scientific experiments. Understanding the boiling point helps researchers maintain accurate temperatures.
Beyond the Boiling Point: Superheating and Subcooling
While 373.15 K is the typical boiling point, it's possible to temporarily exceed this temperature without boiling. This phenomenon is known as superheating. Superheating can occur when the water is heated very carefully, preventing the formation of nucleation sites (tiny imperfections on surfaces where bubbles can form). However, superheated water is unstable and can suddenly and violently boil if disturbed.
Conversely, subcooling occurs when a liquid is cooled below its freezing point without solidifying. This can happen if the liquid is exceptionally pure and free of nucleation sites for ice crystal formation.
Conclusion: A Deeper Understanding of Boiling
The boiling point of water at 373.15 K is more than just a number; it's a fundamental concept reflecting the interplay between temperature, pressure, molecular motion, and phase transitions. Understanding the factors affecting the boiling point extends beyond simply knowing the temperature; it unlocks a deeper appreciation for the principles of thermodynamics and their impact on various aspects of our lives, from cooking to industrial processes and scientific research. This knowledge provides a solid foundation for further exploration of phase transitions, the behavior of fluids, and the intricate world of thermodynamics. Remember that this understanding extends beyond simply memorizing a figure; it involves grasping the dynamic processes behind this fundamental physical phenomenon. By understanding the "why" behind the "what," we can truly appreciate the significance of the boiling point of water.
Latest Posts
Latest Posts
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Temperature Does Water Boil Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
