What Reagent Is Required To Accomplish The Following Transformation
News Leon
Mar 17, 2025 · 6 min read
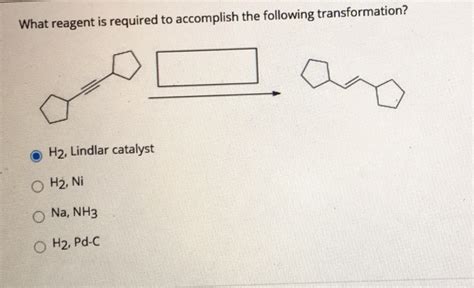
Table of Contents
What Reagent is Required to Accomplish the Following Transformation? A Comprehensive Guide
Choosing the correct reagent for a specific chemical transformation is a cornerstone of organic chemistry. This seemingly simple question – "What reagent is required to accomplish the following transformation?" – often hides a wealth of complexity, requiring a deep understanding of reaction mechanisms, functional group reactivity, and selectivity. This article will explore this question in depth, examining various reaction types and providing a systematic approach to selecting the appropriate reagent. We'll delve into common transformations, discussing the necessary reagents and their mechanisms of action. The goal is to equip you with the tools to confidently tackle such problems.
Understanding the Fundamentals: Reaction Types and Functional Groups
Before diving into specific transformations, it's crucial to understand the fundamental reaction types and functional groups involved. Organic chemistry is built upon a foundation of a few key reaction mechanisms:
-
Addition Reactions: These involve the addition of a molecule across a multiple bond (e.g., alkene, alkyne, carbonyl). Examples include hydrohalogenation, hydration, and halogenation.
-
Substitution Reactions: These involve the replacement of one atom or group with another. Subtypes include SN1, SN2, electrophilic aromatic substitution, and nucleophilic acyl substitution.
-
Elimination Reactions: These involve the removal of atoms or groups from a molecule, often leading to the formation of a multiple bond. Examples include dehydration and dehydrohalogenation.
-
Redox Reactions: These involve the transfer of electrons, resulting in changes in oxidation states. Examples include oxidation of alcohols to aldehydes or ketones, and reduction of ketones to alcohols.
-
Rearrangement Reactions: These involve the reorganization of atoms within a molecule, without the addition or removal of atoms. Examples include Claisen rearrangements and Cope rearrangements.
Knowing the functional groups present in the starting material and the desired product is essential. Common functional groups include alcohols, aldehydes, ketones, carboxylic acids, amines, and halides. Each functional group possesses unique reactivity, dictating the choice of reagent.
A Systematic Approach to Reagent Selection
Solving "What reagent is required...?" problems requires a systematic approach:
-
Identify the Functional Groups: Carefully examine the starting material and the product. What functional groups are present in each? What changes have occurred? This will pinpoint the type of reaction needed (addition, substitution, elimination, etc.).
-
Determine the Reaction Type: Based on the changes in functional groups, determine the type of reaction necessary to achieve the transformation. Is it an oxidation, reduction, addition, substitution, or elimination? Or perhaps a combination of these?
-
Consider Reaction Mechanisms: Understanding the mechanism is critical for choosing the right reagent. For example, SN1 reactions require a carbocation intermediate, while SN2 reactions proceed through a concerted mechanism. Knowing this will help you select a reagent compatible with the desired mechanism.
-
Select the Appropriate Reagent: Based on the reaction type and mechanism, choose the reagent that will selectively and efficiently carry out the transformation. Consider factors such as reaction conditions (temperature, solvent), selectivity, and yield.
-
Evaluate Side Reactions: Many reagents can lead to side reactions, producing unwanted byproducts. Consider the potential for side reactions and choose a reagent that minimizes these possibilities.
-
Check for Stereochemistry: If the transformation involves stereochemistry (chirality), consider the stereochemical outcome of the reaction. Some reagents are stereospecific, while others are stereoselective.
Examples of Reagent Selection in Common Transformations
Let's illustrate this systematic approach with some examples:
Example 1: Conversion of an Alcohol to an Alkyl Halide
- Starting Material: An alcohol (R-OH)
- Product: An alkyl halide (R-X, where X = Cl, Br, I)
- Reaction Type: Substitution
- Reagent Choices: Several reagents can accomplish this transformation. For primary alcohols, thionyl chloride (SOCl2) or phosphorus tribromide (PBr3) are common choices. For secondary and tertiary alcohols, concentrated hydrohalic acids (HCl, HBr, HI) can be used.
Example 2: Oxidation of a Primary Alcohol to an Aldehyde
- Starting Material: A primary alcohol (R-CH2OH)
- Product: An aldehyde (R-CHO)
- Reaction Type: Oxidation
- Reagent Choices: Pyridinium chlorochromate (PCC) is a mild oxidizing agent that selectively oxidizes primary alcohols to aldehydes, avoiding further oxidation to carboxylic acids. Other options include Swern oxidation (using DMSO, oxalyl chloride, and a base) or Dess-Martin periodinane.
Example 3: Conversion of an Alkene to an Alkane
- Starting Material: An alkene (R-CH=CH-R)
- Product: An alkane (R-CH2-CH2-R)
- Reaction Type: Reduction (hydrogenation)
- Reagent Choices: Hydrogen gas (H2) in the presence of a metal catalyst such as platinum (Pt), palladium (Pd), or nickel (Ni) is commonly used for alkene hydrogenation.
Example 4: Grignard Reaction
- Starting Material: An alkyl halide (R-X)
- Product: An alcohol (R-OH) after reacting with a carbonyl compound
- Reaction Type: Nucleophilic addition
- Reagent Choices: A Grignard reagent is formed by reacting an alkyl halide with magnesium metal in anhydrous ether. The Grignard reagent then acts as a nucleophile, attacking the carbonyl carbon of an aldehyde or ketone to eventually form an alcohol after an acid workup.
Example 5: Wittig Reaction
- Starting Material: An aldehyde or ketone
- Product: An alkene
- Reaction Type: Formation of a carbon-carbon double bond
- Reagent Choices: A Wittig reagent (phosphorus ylide) is reacted with an aldehyde or ketone to form an alkene. The specific ylide determines the structure of the alkene formed.
Advanced Considerations and Special Cases
The examples above represent common transformations. However, many other reactions and reagents exist, often tailored for specific substrates or reaction conditions. Some advanced considerations include:
-
Protecting Groups: Protecting groups are used to temporarily mask reactive functional groups during a multi-step synthesis, preventing unwanted reactions. Choosing the appropriate protecting group is crucial for complex syntheses.
-
Regioselectivity and Stereoselectivity: Many reagents exhibit regioselectivity (preference for reaction at a specific site) or stereoselectivity (preference for formation of a specific stereoisomer). Understanding these selectivities is crucial for obtaining the desired product.
-
Solvent Effects: The choice of solvent can significantly influence reaction rates and selectivities. Careful selection of solvent is essential for optimal results.
-
Catalysis: Catalysts are often used to accelerate reactions or improve selectivities. Various catalysts are available, each with its advantages and disadvantages.
Conclusion
Choosing the correct reagent for a specific chemical transformation is a multifaceted process requiring a deep understanding of organic chemistry principles. This article has provided a framework for approaching such problems systematically. By carefully considering the functional groups, reaction types, mechanisms, and potential side reactions, you can confidently select the appropriate reagent for your desired transformation. Remember to consult reliable organic chemistry textbooks and resources for detailed information on specific reagents and reaction conditions. Consistent practice and problem-solving are key to mastering this essential skill. As your understanding deepens, you'll develop an intuitive sense for reagent selection, making the process more efficient and effective.
Latest Posts
Latest Posts
-
How Many Oxygen Molecules Can One Hemoglobin Carry
Mar 18, 2025
-
Which Of The Following Is Not A Form Of Precipitation
Mar 18, 2025
-
Which Statement About Natural Selection Is True
Mar 18, 2025
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Reagent Is Required To Accomplish The Following Transformation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
