What Occupies Space And Has Mass
News Leon
Apr 06, 2025 · 6 min read
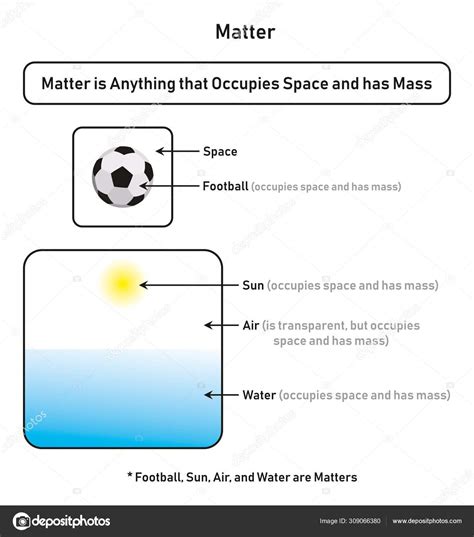
Table of Contents
What Occupies Space and Has Mass: Exploring Matter and Its Properties
The fundamental question, "What occupies space and has mass?" leads us directly to the concept of matter. Matter is anything that occupies space and has mass. This seemingly simple definition underpins our understanding of the universe, from the smallest subatomic particles to the largest galaxies. This article delves deep into the nature of matter, exploring its various forms, properties, and the scientific principles that govern its behavior.
Understanding Mass and Space
Before we delve into the specifics of matter, let's clarify the two key properties that define it: mass and space.
Mass: A Measure of Inertia
Mass is a fundamental property of matter that represents its resistance to acceleration. In simpler terms, it's a measure of how much "stuff" is in an object. A heavier object has more mass and requires more force to change its velocity than a lighter object. Mass is often confused with weight, but they are distinct concepts. Weight is the force of gravity acting on an object's mass. An object's mass remains constant regardless of its location, while its weight can vary depending on the gravitational field.
Space: The Three-Dimensional Extent
Space refers to the three-dimensional expanse in which objects exist and move. It's the volume an object occupies. Understanding space is crucial because matter fills space. The amount of space an object occupies is its volume. The relationship between mass and volume is density, a crucial property in understanding different types of matter.
The Forms of Matter: From Atoms to Galaxies
Matter exists in various forms, ranging from the incredibly small to the astronomically large. These forms are often categorized based on their physical state and chemical composition.
1. Solids: Fixed Shape and Volume
Solids are characterized by their rigid structure and fixed shape and volume. The particles within a solid are closely packed together and held in place by strong intermolecular forces. This restricts their movement, resulting in the solid's stability and resistance to deformation. Examples include rocks, ice, wood, and metals. Different solids can exhibit various properties, such as hardness, brittleness, malleability, and ductility, depending on the arrangement and bonding of their constituent particles.
2. Liquids: Fixed Volume, Variable Shape
Liquids have a fixed volume but take the shape of their container. The particles in a liquid are more loosely packed than in a solid, allowing them to move more freely. This fluidity enables liquids to flow and conform to the shape of their container, while still maintaining a consistent volume. Water, oil, and mercury are common examples. The viscosity of a liquid, its resistance to flow, depends on the intermolecular forces between its particles.
3. Gases: Variable Shape and Volume
Gases have neither a fixed shape nor a fixed volume. Their particles are widely dispersed and move rapidly in random directions. This allows gases to expand to fill any container they occupy. Air, oxygen, and carbon dioxide are examples of gases. The pressure of a gas is a measure of the force exerted by its particles on the walls of its container, influenced by temperature and the number of particles present.
4. Plasma: Ionized Gas
Plasma is often considered the fourth state of matter. It's a highly energized state of matter where atoms are stripped of their electrons, forming ions and free electrons. This creates a highly electrically conductive medium. Plasma is found in stars, lightning bolts, and fluorescent lights. Its unique properties allow it to respond strongly to electromagnetic fields and exhibit behaviors distinct from solids, liquids, and gases.
5. Bose-Einstein Condensates (BEC): A Quantum State of Matter
At extremely low temperatures, some atoms can lose their individual identities and merge into a single quantum entity known as a Bose-Einstein condensate. In this state, a large number of atoms occupy the same quantum state, resulting in macroscopic quantum phenomena. BECs represent a fascinating area of physics exploring the interface between quantum mechanics and the macroscopic world.
The Building Blocks of Matter: Atoms and Molecules
The fundamental building blocks of all matter are atoms. Atoms are the smallest units of an element that retain the chemical properties of that element. They consist of a central nucleus containing protons and neutrons, surrounded by orbiting electrons. The number of protons defines the element's atomic number and its position on the periodic table.
Atoms combine to form molecules. A molecule is a group of two or more atoms held together by chemical bonds. Molecules can be composed of atoms of the same element (like oxygen, O2) or different elements (like water, H2O). The properties of a molecule are determined by the types of atoms it contains and the way they are bonded together.
Properties of Matter: Intensive and Extensive
Matter exhibits a wide range of properties that can be categorized as either intensive or extensive.
Intensive Properties: Independent of Amount
Intensive properties are independent of the amount of matter present. Examples include:
- Density: Mass per unit volume.
- Temperature: A measure of the average kinetic energy of particles.
- Melting point: The temperature at which a solid transitions to a liquid.
- Boiling point: The temperature at which a liquid transitions to a gas.
- Color: The wavelength of light reflected by a substance.
Extensive Properties: Dependent on Amount
Extensive properties depend on the amount of matter present. Examples include:
- Mass: The amount of matter in an object.
- Volume: The amount of space an object occupies.
- Length: The distance between two points on an object.
- Heat capacity: The amount of heat required to raise the temperature of a substance by a certain degree.
States of Matter and Phase Transitions
The transition between different states of matter is called a phase transition. These transitions involve energy changes, as energy is absorbed or released during the process. For example:
- Melting: Solid to liquid (energy absorbed)
- Freezing: Liquid to solid (energy released)
- Vaporization (boiling or evaporation): Liquid to gas (energy absorbed)
- Condensation: Gas to liquid (energy released)
- Sublimation: Solid to gas (energy absorbed)
- Deposition: Gas to solid (energy released)
The Importance of Understanding Matter
Understanding what occupies space and has mass is crucial for numerous scientific disciplines and technological advancements. Knowledge of matter's properties allows us to:
- Develop new materials: By manipulating the atomic and molecular structure of matter, we can create materials with specific properties for various applications.
- Design efficient energy systems: Understanding the behavior of matter at different temperatures and pressures is essential for developing efficient energy storage and conversion technologies.
- Advance medical science: Understanding the structure and function of biological molecules is crucial for developing new drugs and treatments.
- Explore the universe: Studying the matter in space, from stars to galaxies, provides insights into the origin and evolution of the universe.
Conclusion: The Ever-Evolving Understanding of Matter
The simple question of what occupies space and has mass leads us on a fascinating journey into the heart of physics and chemistry. From the fundamental particles that make up atoms to the complex structures that form galaxies, matter continues to surprise and challenge our understanding. As scientific research advances, our knowledge of matter's properties and behavior will continue to expand, driving innovation and shaping our future. The exploration of matter is an ongoing process, and with each new discovery, we deepen our understanding of the universe and our place within it. Further research into dark matter and dark energy, which constitute a significant portion of the universe's mass and energy, promises to further revolutionize our understanding of what occupies space and has mass. The study of matter is not just a scientific pursuit; it’s a journey of discovery that continues to unveil the mysteries of the cosmos.
Latest Posts
Latest Posts
-
Which Is Characteristic Of All Mixtures
Apr 07, 2025
-
How To Factor 2x 2 7x 3
Apr 07, 2025
-
2 3 3 Trimethylbut 1 Ene
Apr 07, 2025
-
Which Of The Following Compounds Is Aromatic
Apr 07, 2025
-
What Is The Ratio Of Carbon To Hydrogen To Oxygen
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Occupies Space And Has Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.