Which Is Characteristic Of All Mixtures
News Leon
Apr 07, 2025 · 6 min read
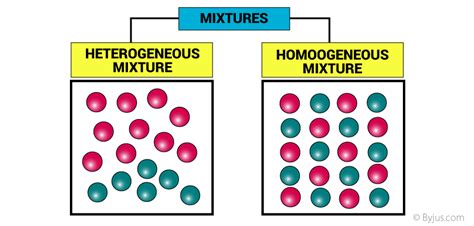
Table of Contents
- Which Is Characteristic Of All Mixtures
- Table of Contents
- Which Characteristic is Common to All Mixtures?
- The Defining Feature: Physical Combination, Not Chemical Bonding
- Exploring the Diverse World of Mixtures
- 1. Homogeneous Mixtures (Solutions): A Uniform Blend
- 2. Heterogeneous Mixtures: A Visible Variety
- Suspensions and Colloids: A Closer Look at Heterogeneous Mixtures
- Understanding the Properties of Mixtures
- Mixtures in Everyday Life and Industry
- Separating Mixtures: Techniques and Applications
- Conclusion: The Ubiquitous Nature of Mixtures
- Latest Posts
- Latest Posts
- Related Post
Which Characteristic is Common to All Mixtures?
The seemingly simple question, "Which characteristic is common to all mixtures?" leads to a fascinating exploration of matter and its diverse forms. Understanding the defining features of mixtures is crucial in various scientific fields, from chemistry and materials science to environmental studies and even cooking. While the specific composition of mixtures varies enormously, a single, unifying characteristic holds true for all of them: mixtures are composed of two or more substances that are physically combined, not chemically bonded. This seemingly straightforward statement unlocks a deeper understanding of the properties and behaviors of mixtures.
The Defining Feature: Physical Combination, Not Chemical Bonding
Let's delve deeper into this core characteristic. Unlike compounds, where atoms of different elements are chemically bonded to form a new substance with distinct properties, mixtures retain the individual properties of their constituent components. This is because the components are only physically combined – they haven't undergone a chemical reaction that alters their fundamental nature. The forces holding the components together in a mixture are weaker intermolecular forces, not strong chemical bonds.
This crucial difference manifests in several key ways:
-
Retention of Individual Properties: In a mixture of sand and iron filings, for instance, you can still identify the individual grains of sand and the metallic iron filings. Their physical properties—color, texture, magnetism—remain unchanged. This contrasts sharply with a chemical compound, where the properties of the constituent elements are lost as a new substance is formed.
-
Variable Composition: Mixtures can have varying compositions. You could have a mixture of sand and iron filings with a 50/50 ratio, or a mixture with 90% sand and 10% iron filings. The proportions are not fixed, unlike in a chemical compound where the ratio of elements is always constant.
-
Separation by Physical Means: This is a defining characteristic. The components of a mixture can be separated using physical methods, such as filtration, distillation, evaporation, magnetism, or chromatography. No chemical reaction is necessary. For example, you can easily separate the sand and iron filings using a magnet.
Exploring the Diverse World of Mixtures
Mixtures exhibit a remarkable diversity, categorized primarily by the size and distribution of their components:
1. Homogeneous Mixtures (Solutions): A Uniform Blend
Homogeneous mixtures, also known as solutions, appear uniform throughout. At a macroscopic level, you cannot distinguish the individual components. The components are evenly dispersed at a molecular or ionic level. Examples include:
- Saltwater: Salt (NaCl) dissolves completely in water, forming a homogeneous solution where the sodium and chloride ions are evenly distributed among the water molecules.
- Air: Air is a mixture of various gases (nitrogen, oxygen, argon, carbon dioxide, etc.) uniformly distributed.
- Brass: This alloy is a homogeneous mixture of copper and zinc.
2. Heterogeneous Mixtures: A Visible Variety
Heterogeneous mixtures show visible differences in composition. You can easily distinguish the different components with the naked eye or with a simple magnifying glass. Examples include:
- Sand and Water: The sand particles are clearly visible in the water.
- Oil and Water: These two liquids do not mix and form distinct layers.
- Granite: This rock is a heterogeneous mixture of different minerals like quartz, feldspar, and mica.
- Concrete: A composite material made of cement, aggregate (sand, gravel), and water.
Suspensions and Colloids: A Closer Look at Heterogeneous Mixtures
Within the category of heterogeneous mixtures, we find suspensions and colloids, characterized by the size of the dispersed particles:
-
Suspensions: These contain relatively large particles that settle out over time if left undisturbed. Examples include muddy water (where the mud particles eventually settle at the bottom) and a mixture of sand and water.
-
Colloids: Colloids have particles of intermediate size that remain suspended indefinitely. They exhibit the Tyndall effect, scattering light, creating a cloudy appearance. Examples include milk (where fat globules are dispersed in water), fog (water droplets in air), and mayonnaise (oil droplets in water).
Understanding the Properties of Mixtures
The properties of mixtures are determined by the properties of their individual components and their relative proportions. This differs significantly from compounds where the properties are entirely new and distinct from the constituents.
Some key properties include:
-
Density: The density of a mixture is usually an average of the densities of its components, weighted by their proportions.
-
Boiling Point and Melting Point: Mixtures typically have a range of boiling and melting points, unlike pure substances which have sharp, defined points.
-
Solubility: The solubility of one component in another significantly influences the properties and behavior of the mixture.
-
Color and Appearance: These properties often reflect the properties of the individual components and their distribution within the mixture.
Mixtures in Everyday Life and Industry
Mixtures are ubiquitous in our daily lives and in various industries. A few examples:
-
Food: Most foods are mixtures, ranging from simple mixtures like cereal and milk to more complex combinations such as cakes and soups.
-
Medicine: Many medicines are mixtures of active ingredients and excipients (inactive substances) that aid in the delivery and absorption of the drug.
-
Cosmetics: Creams, lotions, and makeup are often complex mixtures of various ingredients.
-
Construction Materials: Concrete, asphalt, and many other construction materials are mixtures designed for specific properties.
-
Environmental Science: Understanding the composition of mixtures in air, water, and soil is crucial for environmental monitoring and pollution control.
Separating Mixtures: Techniques and Applications
The ability to separate the components of a mixture is fundamental in many scientific and industrial processes. Common techniques include:
-
Filtration: Separates solids from liquids using a porous material (like filter paper).
-
Distillation: Separates liquids with different boiling points by vaporization and condensation.
-
Evaporation: Separates dissolved solids from a liquid by evaporating the solvent.
-
Crystallization: Separates dissolved solids by allowing them to precipitate out of solution as crystals.
-
Chromatography: Separates components based on their different affinities for a stationary and a mobile phase.
-
Centrifugation: Separates components based on their density using centrifugal force.
-
Magnetic Separation: Separates magnetic materials from non-magnetic materials using a magnet.
Conclusion: The Ubiquitous Nature of Mixtures
In conclusion, the unifying characteristic of all mixtures is their physical combination of two or more substances without chemical bonding. This fundamental property leads to a vast range of properties, compositions, and behaviors. From the air we breathe to the food we eat, mixtures are integral to our world, and understanding their characteristics is essential across numerous scientific disciplines and everyday applications. The ability to separate the components of mixtures is a key skill in many areas, highlighting the ongoing significance of this seemingly simple concept in a complex world. The continued exploration and understanding of mixtures will undoubtedly lead to further advancements in various fields.
Latest Posts
Latest Posts
-
Is Prepaid Rent An Asset Or Liabilities
Apr 11, 2025
-
Arrange The Amines In Order Of Boiling Point
Apr 11, 2025
-
An Emulsion Is Classified As A Specific Type Of
Apr 11, 2025
-
What Are Alternate Forms Of A Gene Called
Apr 11, 2025
-
An Adjective Can Describe Both A
Apr 11, 2025
Related Post
Thank you for visiting our website which covers about Which Is Characteristic Of All Mixtures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.