What Is The Substrate For Lipase
News Leon
Mar 28, 2025 · 6 min read
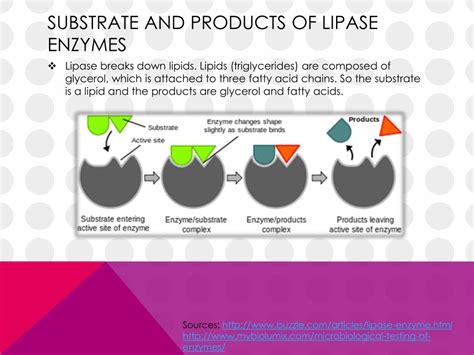
Table of Contents
What is the Substrate for Lipase? A Deep Dive into Enzyme Specificity and Applications
Lipases, a class of hydrolytic enzymes, are ubiquitous in nature, playing crucial roles in diverse biological processes. Understanding their substrates is fundamental to comprehending their function and harnessing their potential in various applications. This article delves into the intricacies of lipase substrates, exploring their structural features, the enzyme's specificity, and the implications for industrial and biotechnological applications.
Defining Lipase and its Function
Lipases (EC 3.1.1.3) are serine hydrolases that catalyze the hydrolysis of fats (lipids). More specifically, they break down triglycerides, the primary form of dietary fat, into glycerol and free fatty acids. This hydrolysis occurs at the interface between the aqueous phase and the lipid phase, a characteristic feature distinguishing them from other esterases. Their primary function, biologically, is the digestion and metabolism of fats, enabling organisms to utilize the energy stored within lipids.
The Nature of Lipase Substrates: Triglycerides and Beyond
While triglycerides are the most well-known substrates, lipase activity extends to a wider range of substrates. Let's explore these:
Triglycerides: The Primary Substrate
Triglycerides, also known as triacylglycerols, are the predominant lipids found in animal and plant tissues. They consist of a glycerol backbone esterified to three fatty acid chains. The length, saturation, and degree of unsaturation of these fatty acid chains significantly influence lipase activity. Different lipases exhibit varying preferences for specific fatty acid compositions. For example, some lipases show a higher affinity for short-chain fatty acids, while others prefer long-chain unsaturated fatty acids.
Fatty Acid Chain Length: A Key Determinant
The length of the fatty acid chain significantly impacts lipase activity. Short-chain fatty acids (SCFA) generally result in faster hydrolysis rates compared to long-chain fatty acids (LCFA). The reason lies in the accessibility of the ester bond to the enzyme's active site. Shorter chains allow for easier access and interaction. Very long-chain fatty acids (VLCFA) might even hinder enzyme activity due to steric hindrance.
Fatty Acid Saturation and Unsaturation: Impact on Substrate Binding
The degree of saturation (presence of double bonds) in the fatty acid chain is another crucial factor. Lipases often exhibit selectivity towards specific levels of unsaturation. Some lipases preferentially hydrolyze saturated fatty acids, while others favor unsaturated fatty acids. The presence of cis or trans double bonds can further influence substrate binding and enzyme kinetics. The conformation of the fatty acid chain in the substrate influences its accessibility to the enzyme’s active site.
Positional Specificity: A Matter of Preference
Another aspect of lipase substrate specificity is positional specificity. This refers to the preference for hydrolyzing ester bonds at specific positions on the glycerol backbone. Some lipases are 1,3-specific, meaning they preferentially hydrolyze the ester bonds at the sn-1 and sn-3 positions, leaving the sn-2 position relatively untouched. Others are sn-2 specific, while some show non-specific activity, hydrolyzing all three positions. This positional specificity is determined by the enzyme's active site structure and its interaction with the substrate.
Beyond Triglycerides: Expanding the Substrate Scope
While triglycerides dominate the discussion, lipases exhibit activity toward other substrates, including:
-
Phospholipids: Certain lipases can hydrolyze phospholipids, which are crucial components of cell membranes. This activity is relevant in various physiological processes and in biotechnological applications involving membrane manipulation.
-
Cholesterol esters: Some lipases display activity towards cholesterol esters, which are involved in cholesterol transport and metabolism. This activity is particularly important in the context of cardiovascular health and related research.
-
Other esters: Lipases can also hydrolyze other types of esters, including those found in waxes and other lipids. This versatility makes lipases valuable tools in various industrial settings.
-
Synthetic Esters: The catalytic versatility of lipases extends to synthetic esters as well. These could include esters with different alcohols and/or modified fatty acids, opening up possibilities for tailored reactions and specific product synthesis.
Factors Influencing Lipase Activity and Substrate Binding
Several factors beyond substrate structure influence lipase activity and substrate binding:
-
Temperature: Lipases exhibit optimal activity within a specific temperature range. High temperatures can denature the enzyme, while low temperatures can reduce catalytic efficiency.
-
pH: Similar to temperature, lipases have an optimal pH range for activity. Variations in pH can alter the enzyme's conformation, affecting substrate binding and catalytic activity.
-
Solvent: The solvent used in lipase-catalyzed reactions significantly impacts enzyme activity. Aqueous solutions are generally preferred for hydrolysis reactions, but some lipases exhibit activity in organic solvents. The choice of solvent influences substrate solubility and enzyme stability.
-
Enzyme Concentration: The concentration of lipase in the reaction mixture affects the rate of reaction. Increasing enzyme concentration generally increases reaction speed, until a saturation point is reached.
-
Substrate Concentration: The concentration of the substrate also influences reaction kinetics. Increasing substrate concentration increases the reaction rate up to a point, beyond which the enzyme becomes saturated.
Industrial and Biotechnological Applications Leveraging Lipase Substrate Specificity
The diverse substrate specificity of lipases makes them valuable tools in a wide array of industrial and biotechnological applications:
-
Food Industry: Lipases are extensively used in food processing for the production of various food products, including cheese, butter, and other dairy products. They are also used in the modification of fats and oils to improve their texture, flavor, and nutritional properties. Lipase-catalyzed hydrolysis and transesterification reactions are key in modifying the fatty acid profile of products to achieve desirable characteristics.
-
Biofuel Production: Lipases are employed in the production of biodiesel, a renewable alternative to fossil fuels. They catalyze the transesterification of vegetable oils and animal fats with methanol or ethanol to produce biodiesel and glycerol. The efficiency and specificity of the lipases used play a pivotal role in the overall economics and sustainability of biodiesel production.
-
Pharmaceutical Industry: Lipases are used in the synthesis of various pharmaceuticals, including chiral drugs and other complex molecules. Their stereospecificity makes them crucial in the production of enantiomerically pure compounds.
-
Detergent Industry: Lipases are incorporated into laundry detergents to aid in the removal of grease and oily stains from fabrics. Their ability to hydrolyze lipids makes them highly effective in this application.
-
Cosmetic Industry: Lipases are increasingly being utilized in cosmetic applications, specifically in the production of skincare products and emulsifiers. Their gentle action on lipids makes them suitable for delicate formulations.
Future Directions in Lipase Research
Ongoing research focuses on improving the efficiency and specificity of lipases for various applications. This involves:
-
Enzyme Engineering: Modifying lipases through genetic engineering techniques to enhance their activity towards specific substrates or under specific reaction conditions.
-
Directed Evolution: Using evolutionary methods to select and enhance lipases with desired properties.
-
Immobilization Techniques: Developing efficient methods to immobilize lipases on solid supports, improving their stability and reusability in industrial processes.
-
Computational Modeling: Utilizing computational tools to design and predict the activity of novel lipases.
Conclusion
The substrate for lipase extends beyond just triglycerides, encompassing a range of lipids and esters. Understanding the intricacies of lipase-substrate interactions, including fatty acid chain length, saturation, position on the glycerol backbone, and other factors affecting enzyme activity, is paramount for exploiting the full potential of these versatile biocatalysts. The continued exploration of lipase specificity and the development of novel enzyme engineering strategies pave the way for innovative applications across multiple industries, leading to more sustainable and efficient processes. The versatility and specificity of lipases are continuously being harnessed to improve existing technologies and drive the development of new ones, solidifying their importance in biotechnology and industrial processes.
Latest Posts
Latest Posts
-
Blocks A And B Of Masses Ma And Mb
Mar 31, 2025
-
The Height Above Sea Level Is Called
Mar 31, 2025
-
For What Value Of X Is Abc Def
Mar 31, 2025
-
Which Elements Have 5 Valence Electrons
Mar 31, 2025
-
Which Of The Following Is An Example Of Nonvolatile Memory
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Substrate For Lipase . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
