What Is The Ph Of Neutral Solution
News Leon
Mar 27, 2025 · 6 min read
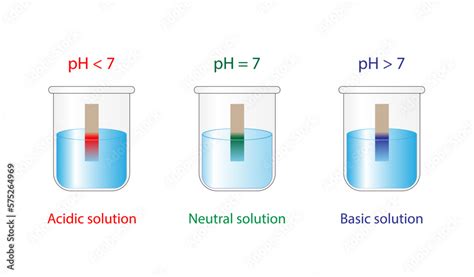
Table of Contents
What is the pH of a Neutral Solution? A Deep Dive into pH, Acidity, and Alkalinity
The concept of pH is fundamental to chemistry and numerous applications across various scientific disciplines and everyday life. Understanding pH is crucial for comprehending chemical reactions, biological processes, and environmental conditions. This comprehensive article delves into the meaning of pH, specifically addressing the question: What is the pH of a neutral solution? We'll explore the pH scale, factors affecting pH, and the significance of neutrality in different contexts.
Understanding the pH Scale
The pH scale is a logarithmic scale used to specify the acidity or basicity (alkalinity) of an aqueous solution. It ranges from 0 to 14, with 7 representing neutrality.
- pH < 7: Indicates an acidic solution. The lower the pH value, the stronger the acid.
- pH = 7: Indicates a neutral solution.
- pH > 7: Indicates an alkaline (basic) solution. The higher the pH value, the stronger the base.
The scale is logarithmic, meaning each whole number change represents a tenfold change in the concentration of hydrogen ions (H⁺). For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and one hundred times more acidic than a solution with a pH of 5.
The Role of Hydrogen Ions (H⁺) and Hydroxide Ions (OH⁻)
The pH of a solution is determined by the relative concentrations of hydrogen ions (H⁺) and hydroxide ions (OH⁻). Pure water undergoes a process called autoionization, where a small fraction of water molecules dissociate into H⁺ and OH⁻ ions:
2H₂O ⇌ H₃O⁺ + OH⁻
In pure water, the concentrations of H⁺ and OH⁻ are equal, resulting in a neutral pH. This equilibrium is crucial in maintaining the balance of acidity and basicity.
What Determines the pH of a Solution?
Several factors can influence the pH of a solution:
-
Concentration of H⁺ and OH⁻ ions: As mentioned, the primary determinant of pH is the ratio of H⁺ to OH⁻ ions. Strong acids readily dissociate, releasing a high concentration of H⁺ ions, resulting in a low pH. Strong bases release a high concentration of OH⁻ ions, leading to a high pH.
-
Temperature: Temperature affects the autoionization of water. At higher temperatures, the concentration of both H⁺ and OH⁻ ions increases, but the pH of pure water remains at 7 because the concentrations remain equal. However, the pH of other solutions might shift slightly with temperature changes due to variations in the dissociation constants of acids and bases.
-
Presence of Buffers: Buffers are solutions that resist changes in pH when small amounts of acid or base are added. They consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. Buffers are crucial in maintaining a stable pH in biological systems.
-
Dissolution of Salts: Some salts, when dissolved in water, can affect the pH of the solution. Salts derived from strong acids and weak bases produce acidic solutions, while salts derived from strong bases and weak acids produce basic solutions. Salts derived from strong acids and strong bases produce neutral solutions.
The pH of a Neutral Solution: A Deeper Look
A neutral solution has a pH of 7 at 25°C (77°F). This is because the concentration of hydrogen ions (H⁺) is equal to the concentration of hydroxide ions (OH⁻). In pure water, this concentration is approximately 1 x 10⁻⁷ moles per liter. The equilibrium constant for the autoionization of water (Kw) is the product of the H⁺ and OH⁻ concentrations:
Kw = [H⁺][OH⁻] = 1 x 10⁻¹⁴ at 25°C
Since [H⁺] = [OH⁻] in a neutral solution, we have:
[H⁺] = [OH⁻] = √Kw = 1 x 10⁻⁷ M
This means that the concentration of hydrogen ions and hydroxide ions are both 1 x 10⁻⁷ moles per liter in a neutral solution at 25°C.
Deviation from Neutrality: The Importance of Temperature
It's crucial to note that the pH of a neutral solution is temperature-dependent. At temperatures above 25°C, the Kw value increases, meaning the concentrations of both H⁺ and OH⁻ increase. However, they remain equal, so the pH of pure water remains 7. While the pH of pure water always remains at 7, other solutions may experience slight pH shifts with temperature changes. For example, the pH of some acidic or alkaline solutions may decrease or increase, respectively, with increasing temperature.
Applications and Significance of Neutral pH
Maintaining a neutral pH is essential in numerous applications:
-
Biology: Many biological processes, such as enzyme activity and cellular function, are highly sensitive to pH changes. The pH of blood, for instance, is carefully regulated near neutrality (approximately 7.4) to ensure optimal physiological function. Significant deviations from this range can be life-threatening.
-
Environmental Science: The pH of soil and water plays a critical role in determining the survival and growth of plants and aquatic organisms. Acid rain, for example, can drastically lower the pH of lakes and rivers, harming aquatic life.
-
Industry: Many industrial processes require specific pH ranges for optimal performance. For example, the production of certain chemicals or materials might necessitate maintaining a neutral or slightly acidic/alkaline pH.
-
Everyday Life: The pH of many everyday products, such as shampoos, soaps, and cleaning solutions, is carefully controlled to ensure effectiveness and safety for human use.
Measuring pH
Several methods exist for measuring pH:
-
pH Indicators: These are substances that change color depending on the pH of the solution. Litmus paper is a common example, turning red in acidic solutions and blue in alkaline solutions. More sophisticated pH indicators can provide a more precise range of pH values.
-
pH Meters: These electronic devices use electrodes to measure the voltage difference between a reference electrode and a pH-sensitive electrode. This voltage difference is directly proportional to the pH of the solution. pH meters are generally more accurate than pH indicators.
-
Spectrophotometry: This method utilizes the absorbance of light by a solution to determine the concentration of H⁺ ions and calculate the pH.
Conclusion
The pH of a neutral solution is 7 at 25°C, reflecting the equal concentrations of hydrogen and hydroxide ions. This neutrality is a crucial concept in chemistry, biology, environmental science, and countless other fields. Understanding the pH scale and the factors that influence pH is essential for interpreting and controlling chemical reactions and biological processes. The measurement of pH, using various techniques, allows scientists and researchers to monitor and manipulate pH in a wide array of applications, ensuring optimal performance and safety in diverse settings. The maintenance of a suitable pH is a fundamental requirement for the stability and functionality of many systems, underscoring the importance of understanding this fundamental concept.
Latest Posts
Latest Posts
-
Economic Growth Is Depicted By
Mar 30, 2025
-
Which Of The Following Is A Conductor
Mar 30, 2025
-
Frame A Sentence Using The Word
Mar 30, 2025
-
The Broadest Taxonomic Group For Classifying Living Organisms Is The
Mar 30, 2025
-
Ca Oh 2 Hcl Balanced Equation
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Ph Of Neutral Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
