What Is The Oxidation State Of S In H2so4
News Leon
Mar 17, 2025 · 5 min read
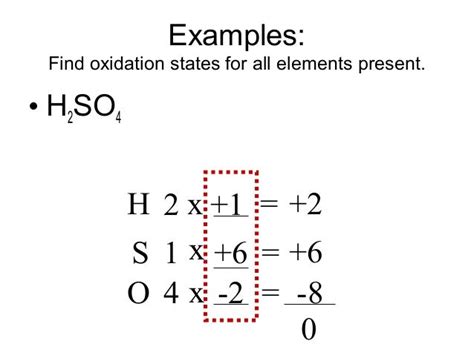
Table of Contents
What is the Oxidation State of S in H₂SO₄? A Deep Dive into Sulfur's Chemistry
Determining the oxidation state of sulfur (S) in sulfuric acid (H₂SO₄) is a fundamental concept in chemistry, crucial for understanding its reactivity and properties. This seemingly simple question opens the door to a fascinating exploration of oxidation states, redox reactions, and the versatile chemistry of sulfur. This comprehensive article will not only answer the question directly but also delve into the underlying principles, providing a thorough understanding for students and enthusiasts alike.
Understanding Oxidation States
Before we tackle the specific case of H₂SO₄, let's solidify our understanding of oxidation states. The oxidation state, also known as the oxidation number, is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are completely ionic. It's a useful tool for tracking electron transfer in chemical reactions, particularly redox (reduction-oxidation) reactions.
Key Rules for Assigning Oxidation States:
- Free elements: The oxidation state of an atom in its elemental form is always 0 (e.g., O₂, S₈).
- Monatomic ions: The oxidation state of a monatomic ion is equal to its charge (e.g., Na⁺ = +1, Cl⁻ = -1).
- Group 1 elements: Always +1.
- Group 2 elements: Always +2.
- Hydrogen: Usually +1, except in metal hydrides where it's -1 (e.g., NaH).
- Oxygen: Usually -2, except in peroxides (-1) and superoxides (-1/2).
- Fluorine: Always -1.
- The sum of oxidation states: In a neutral molecule, the sum of the oxidation states of all atoms must equal zero. In a polyatomic ion, the sum must equal the charge of the ion.
Determining the Oxidation State of Sulfur in H₂SO₄
Now, let's apply these rules to sulfuric acid (H₂SO₄). The molecule is neutral, meaning the sum of the oxidation states must be zero. We know the following:
- Hydrogen (H): Each hydrogen atom typically has an oxidation state of +1. There are two hydrogen atoms, contributing a total of +2.
- Oxygen (O): Each oxygen atom typically has an oxidation state of -2. There are four oxygen atoms, contributing a total of -8.
Let's represent the oxidation state of sulfur as 'x'. According to the rule that the sum of oxidation states in a neutral molecule must equal zero, we can set up the following equation:
(+2) + x + (-8) = 0
Solving for x:
x = +6
Therefore, the oxidation state of sulfur (S) in H₂SO₄ is +6.
The Significance of the +6 Oxidation State
The +6 oxidation state of sulfur in H₂SO₄ highlights several important aspects of its chemistry:
-
High oxidation state implies strong oxidizing power: A high positive oxidation state indicates that sulfur has a strong tendency to gain electrons and undergo reduction. This makes sulfuric acid a strong oxidizing agent, capable of oxidizing many other substances. This is evidenced by its use in various chemical processes requiring strong oxidizing conditions.
-
Stability of the Sulfate Ion (SO₄²⁻): The +6 oxidation state of sulfur contributes to the exceptional stability of the sulfate ion (SO₄²⁻). This tetrahedral ion is a common and stable anion in many chemical compounds and plays a crucial role in various biological processes.
-
Acidic nature of H₂SO₄: The high oxidation state of sulfur contributes to the strong acidic nature of H₂SO₄. The highly electronegative oxygen atoms draw electron density away from the sulfur atom, increasing the polarity of the S-O bonds and facilitating the release of protons (H⁺).
Sulfur's Variable Oxidation States: A Versatile Element
Sulfur, unlike some other elements, exhibits a wide range of oxidation states, ranging from -2 to +6. This versatility is a key factor in its diverse chemical behavior and its role in various important compounds and processes:
- -2 oxidation state: Found in hydrogen sulfide (H₂S) and many metal sulfides. In these compounds, sulfur acts as a reducing agent.
- 0 oxidation state: Present in elemental sulfur (S₈).
- +2 oxidation state: Found in sulfur dioxide (SO₂), a significant air pollutant.
- +4 oxidation state: Found in sulfurous acid (H₂SO₃) and sulfites.
- +6 oxidation state: Found in sulfuric acid (H₂SO₄) and sulfates, a vital component of many fertilizers.
Redox Reactions Involving H₂SO₄
Sulfuric acid's strong oxidizing power is central to its role in numerous redox reactions. For example:
-
Reaction with metals: Concentrated sulfuric acid can oxidize many metals, such as copper (Cu), producing sulfur dioxide (SO₂) and metal sulfates. The balanced equation for the reaction with copper is:
Cu(s) + 2H₂SO₄(aq) → CuSO₄(aq) + SO₂(g) + 2H₂O(l)
-
Reaction with reducing agents: H₂SO₄ can oxidize other reducing agents like hydrogen iodide (HI), converting iodide ions (I⁻) to iodine (I₂).
-
Industrial applications: The oxidizing power of sulfuric acid is exploited in many industrial processes, including the production of other chemicals, metal refining, and battery manufacturing.
Beyond H₂SO₄: Other Sulfates and Sulfur Compounds
The +6 oxidation state of sulfur is not unique to sulfuric acid. It is found in many other important sulfates, including:
- Calcium sulfate (CaSO₄): A major component of gypsum and plaster of Paris.
- Magnesium sulfate (MgSO₄): Commonly known as Epsom salt, used in medicine and agriculture.
- Sodium sulfate (Na₂SO₄): Used in the production of detergents and paper.
- Metal sulfates in batteries: Many metal sulfates serve as electrolytes in various battery types.
Conclusion: The Importance of Oxidation States in Understanding Chemistry
Determining the oxidation state of sulfur in H₂SO₄, and understanding the broader concept of oxidation states, is critical for comprehending the chemical behavior of compounds and predicting the outcome of reactions. Sulfuric acid, with its sulfur in the +6 oxidation state, serves as a prime example of how oxidation states influence the reactivity, properties, and applications of a compound. The wide range of oxidation states exhibited by sulfur underscores its versatility as an element, making it essential to various natural and industrial processes. This detailed analysis provides a firm foundation for further exploration of sulfur chemistry and redox reactions. Further research into specific redox reactions involving H₂SO₄ and the detailed mechanisms of these reactions would provide even deeper insights into the fascinating world of sulfur's chemical behavior.
Latest Posts
Latest Posts
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidation State Of S In H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
