What Is The Name Of The Following Compound
News Leon
Apr 06, 2025 · 7 min read
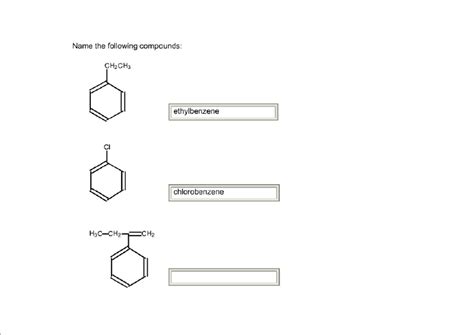
Table of Contents
What's the Name of This Compound? A Deep Dive into IUPAC Nomenclature
Naming chemical compounds might seem like a daunting task, especially when dealing with complex structures. However, understanding the system of nomenclature, specifically the International Union of Pure and Applied Chemistry (IUPAC) system, is crucial for clear communication and unambiguous identification in the world of chemistry. This article delves into the intricacies of IUPAC nomenclature, providing a comprehensive guide to naming various types of compounds and addressing common challenges. We'll equip you with the tools to confidently name and identify countless chemical compounds.
Without knowing the specific chemical structure, it's impossible to provide the name of the compound. This article will serve as a foundational guide to allow you to name any provided chemical structure, using the IUPAC system. Let's break down the process into manageable steps and different compound classes.
Understanding the Fundamentals of IUPAC Nomenclature
The IUPAC system is a standardized method for naming organic and inorganic compounds. It's based on a logical set of rules, prioritizing clarity and avoiding ambiguity. The fundamental principles include:
-
Parent Chain/Structure: This forms the basis of the name. It's the longest continuous chain of carbon atoms in an organic molecule or the central atom in an inorganic compound.
-
Substituents: These are atoms or groups of atoms attached to the parent chain/structure. Their positions and identities are crucial for precise naming.
-
Numbering: The parent chain/structure is numbered to indicate the position of substituents. Numbering aims to provide the lowest possible numbers for the location of the substituents.
-
Prefixes and Suffixes: Prefixes describe the substituents, while suffixes denote the functional group (the reactive part of the molecule) or the type of compound.
Naming Alkanes: The Foundation of Organic Nomenclature
Alkanes are saturated hydrocarbons (containing only single bonds) and form the backbone for many organic compounds. Naming them provides the foundation for understanding the nomenclature of more complex molecules.
Straight-Chain Alkanes:
These are simple chains of carbon atoms. The first four alkanes have trivial names:
- Methane (CH₄)
- Ethane (C₂H₆)
- Propane (C₃H₈)
- Butane (C₄H₁₀)
From pentane (C₅H₁₂) onwards, the names follow a systematic pattern: pentane, hexane, heptane, octane, nonane, decane, and so on, reflecting the number of carbon atoms.
Branched-Chain Alkanes:
These contain carbon atoms branching off from the main chain. Naming them requires:
-
Identifying the longest continuous carbon chain: This becomes the parent alkane.
-
Numbering the parent chain: Begin numbering from the end that gives the substituents the lowest possible numbers.
-
Identifying and naming the substituents: Alkyl groups (branches) are named by replacing the "-ane" ending of the alkane with "-yl" (e.g., methyl, ethyl, propyl, butyl).
-
Indicating the position of the substituents: Use numbers to show where the substituents are attached to the parent chain. If multiple substituents are identical, use prefixes like di-, tri-, tetra-, etc., to indicate their number. List the substituents alphabetically (ignoring di-, tri-, etc.).
Example:
Consider the compound with the structure: CH₃-CH(CH₃)-CH₂-CH₃
- Longest chain: 4 carbons (butane)
- Numbering: Start from the left to give the substituent the lowest number.
- Substituent: One methyl group on carbon 2.
- Name: 2-methylbutane
Naming Alkenes and Alkynes: Compounds with Multiple Bonds
Alkenes contain carbon-carbon double bonds, and alkynes contain carbon-carbon triple bonds. Their naming follows similar principles as alkanes, with additional considerations for the location of the multiple bonds.
-
Identify the longest carbon chain containing the multiple bond: This is the parent chain.
-
Number the parent chain: Start numbering from the end closest to the multiple bond, giving the multiple bond the lowest possible number.
-
Indicate the position of the multiple bond: Use a number to specify the carbon atom where the double or triple bond begins.
-
Use the suffix "-ene" for alkenes and "-yne" for alkynes: Replace the "-ane" ending of the corresponding alkane.
Example:
CH₂=CH-CH₂-CH₃
- Longest chain: 4 carbons (butane)
- Numbering: Start from the left.
- Multiple bond: Double bond at carbon 1.
- Name: 1-butene
Naming Aromatic Compounds: Benzene and Its Derivatives
Aromatic compounds contain a benzene ring (a six-carbon ring with alternating single and double bonds). Simple derivatives of benzene are named by adding the substituent name as a prefix to "benzene."
Example:
C₆H₅-CH₃ (methylbenzene, commonly called toluene)
For compounds with multiple substituents on the benzene ring, use numbers to indicate their positions (starting from a position that gives the lowest possible numbers) and list the substituents alphabetically. You can also use prefixes like ortho (1,2-), meta (1,3-), and para (1,4-) for disubstituted benzenes.
Naming Alcohols, Aldehydes, Ketones, Carboxylic Acids, and Other Functional Groups
These compounds contain specific functional groups that determine their chemical properties and names. The presence of these functional groups dictates the suffix used in the name.
-
Alcohols (-OH): Replace the "-e" ending of the parent alkane with "-ol." Indicate the position of the hydroxyl group (-OH) using a number.
-
Aldehydes (-CHO): Replace the "-e" ending of the parent alkane with "-al." The aldehyde group is always at the end of the chain, so no number is needed.
-
Ketones (C=O): Replace the "-e" ending of the parent alkane with "-one." Use a number to indicate the position of the carbonyl group (C=O).
-
Carboxylic Acids (-COOH): Replace the "-e" ending of the parent alkane with "-oic acid." The carboxyl group is always at the end of the chain, so no number is needed.
-
Ethers (R-O-R'): Name the two alkyl groups alphabetically, followed by "ether".
Dealing with Complex Structures: A Step-by-Step Approach
When confronted with a complex structure, follow these steps:
-
Identify the parent chain or structure: This is the longest continuous carbon chain or the most important functional group.
-
Identify all substituents and functional groups: Name each one individually.
-
Number the parent chain or structure: Assign numbers to the carbons or atoms in a way that minimizes the numbers used to locate the substituents and functional groups.
-
Arrange substituents alphabetically: List substituents in alphabetical order (ignoring prefixes like di-, tri-, etc.) before the parent name.
-
Combine the information to form the IUPAC name: This includes prefixes for substituents, numbers indicating positions, and the suffix denoting the functional group or the parent structure type.
Common Challenges and Considerations:
-
Stereoisomers: These are molecules with the same molecular formula but different spatial arrangements. Naming them requires specifying the configuration (e.g., cis or trans for alkenes, R or S for chiral centers).
-
Cyclic Compounds: Cyclic compounds are named using prefixes like "cyclo-" before the parent alkane name. Numbering begins at a substituent, proceeding in a direction that gives the lowest possible numbers to other substituents.
-
Polyfunctional Compounds: When multiple functional groups are present, the principal functional group determines the suffix, and other functional groups are treated as substituents (using appropriate prefixes). The order of precedence for functional groups is important. Carboxylic acids have the highest precedence, followed by aldehydes, ketones, alcohols, amines, and so on.
Conclusion: Mastering IUPAC Nomenclature
The IUPAC nomenclature system provides a rigorous and unambiguous method for naming chemical compounds. By systematically applying the rules outlined here, you can confidently name a wide range of organic and inorganic compounds, significantly enhancing your understanding and communication within the field of chemistry. Remember to practice regularly, as mastery of this system takes time and effort. Working through numerous examples will solidify your understanding and prepare you to tackle even the most complex chemical structures with confidence. Further exploration of specialized IUPAC rules for specific classes of compounds will further enhance your expertise. This detailed guide provides a strong foundation for understanding the core principles and successfully naming chemical compounds.
Latest Posts
Latest Posts
-
How Many Electrons In F Subshell
Apr 07, 2025
-
Ligaments Are Bundles Of Elastic And Collagen Fibers That
Apr 07, 2025
-
What Charge Does Sodium Ion Have
Apr 07, 2025
-
This Organelle Sorts And Packages Proteins
Apr 07, 2025
-
A Quadrilateral In Which The Diagonals Bisect Each Other
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Is The Name Of The Following Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
