How Many Electrons In F Subshell
News Leon
Apr 07, 2025 · 6 min read
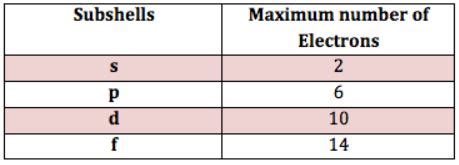
Table of Contents
How Many Electrons in an f Subshell? A Deep Dive into Atomic Structure
The question, "How many electrons in an f subshell?" might seem simple at first glance, but delving into the answer reveals a fascinating journey into the heart of atomic structure and quantum mechanics. Understanding this requires a grasp of fundamental concepts like electron shells, subshells, orbitals, and the Pauli Exclusion Principle. This comprehensive guide will not only answer the question definitively but also explore the underlying principles that govern electron configuration and the periodic table itself.
Understanding Electron Shells and Subshells
Before we tackle the f subshell specifically, let's establish a foundational understanding of electron shells and subshells within an atom. An atom's electrons reside in various energy levels, known as shells. These shells are designated by principal quantum numbers (n), starting with n=1 (the closest to the nucleus) and increasing outward. Each shell can accommodate a specific maximum number of electrons. The formula for the maximum number of electrons in a shell is 2n². Therefore, the first shell (n=1) can hold a maximum of 2 electrons, the second shell (n=2) can hold 8, and so on.
Within each shell are subshells, each characterized by its own angular momentum quantum number (l). These subshells are designated by letters:
- s (l=0): This subshell has a spherical shape and can hold a maximum of 2 electrons.
- p (l=1): This subshell has a dumbbell shape and can hold a maximum of 6 electrons (three orbitals, each holding two electrons).
- d (l=2): This subshell has more complex shapes and can hold a maximum of 10 electrons (five orbitals, each holding two electrons).
- f (l=3): This subshell has even more intricate shapes and can hold a maximum of 14 electrons (seven orbitals, each holding two electrons).
The f Subshell: Orbitals and Electron Capacity
The f subshell, the focus of our exploration, is characterized by its seven orbitals. Each orbital can accommodate a maximum of two electrons, according to the Pauli Exclusion Principle. This principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, m<sub>l</sub>, and m<sub>s</sub>). The magnetic quantum number (m<sub>l</sub>) specifies the orientation of the orbital in space, ranging from -l to +l, including 0. The spin quantum number (m<sub>s</sub>) describes the electron's intrinsic angular momentum, with values of +1/2 (spin up) or -1/2 (spin down).
Therefore, since the f subshell has seven orbitals (m<sub>l</sub> = -3, -2, -1, 0, 1, 2, 3), and each orbital can hold two electrons (one spin up and one spin down), the f subshell can hold a total of 14 electrons.
The Importance of the f Subshell in the Periodic Table
The f subshell plays a crucial role in shaping the periodic table's structure, particularly the lanthanides (rare earth elements) and actinides. These elements are characterized by the filling of the 4f and 5f subshells, respectively. The unique properties of these elements, including their magnetic and spectroscopic behavior, are directly related to the electronic configuration of their f subshells.
The filling of the f subshells doesn't follow a perfectly sequential pattern as predicted by the Aufbau principle (filling orbitals in order of increasing energy). Electron-electron repulsions and other quantum mechanical effects can lead to irregularities in the filling order. This is why the electronic configurations of some lanthanides and actinides might appear somewhat complex and deviate slightly from the expected sequence.
Visualizing the f Subshell
While visualizing the shapes of the f orbitals can be challenging, understanding their number and capacity is key to understanding the electron configurations of elements. The complex shapes arise from the higher angular momentum quantum number (l=3). These orbitals are not simply spherical or dumbbell-shaped like s and p orbitals, but rather have more intricate, multi-lobed structures.
Electron Configuration and the f Subshell
The electron configuration of an atom specifies how electrons are distributed among the various shells and subshells. For example, the electron configuration of Uranium (U), element 92, is [Rn] 5f³ 6d¹ 7s². This notation shows that Uranium has its inner electrons configured like Radon (Rn), and then has 3 electrons in the 5f subshell, 1 in the 6d subshell and 2 in the 7s subshell. Understanding electron configuration is crucial for predicting the chemical and physical properties of elements.
The filling of the f subshell, as mentioned earlier, doesn't always follow a strict order. This leads to subtle variations in the properties of the lanthanides and actinides, creating a rich tapestry of chemical behavior and applications. The irregularities in the filling of f orbitals are a consequence of the complex interplay of quantum mechanical effects that govern electron behavior within the atom.
Applications and Significance of f-Block Elements
The elements with partially or completely filled f subshells (lanthanides and actinides) exhibit unique properties that have numerous applications in various fields. These applications include:
-
Magnets: Many lanthanides are crucial components of powerful permanent magnets, utilized in various technologies from wind turbines to medical imaging devices. Their unique magnetic properties arise from the unpaired electrons in their f subshells.
-
Catalysis: Lanthanide and actinide compounds play a vital role in catalytic processes, acting as catalysts in chemical reactions, such as those involved in petroleum refining and polymerization. Their variable oxidation states and complex coordination chemistry contribute to their catalytic activity.
-
Lighting: Certain lanthanides produce vibrant colors when excited by light, making them indispensable in various lighting applications, including fluorescent lights and high-intensity discharge lamps.
-
Nuclear Technology: Several actinides, notably uranium and plutonium, are essential in nuclear power generation and nuclear weapons technology. Their radioactive properties and capacity for nuclear fission are crucial in these applications.
-
Medical Applications: Some lanthanides find applications in medical imaging (MRI contrast agents) and cancer therapy. Their unique spectroscopic and magnetic properties make them suitable for these roles.
Conclusion: Beyond the Simple Answer
The simple answer to "How many electrons in an f subshell?" is 14. However, understanding this answer requires a deep dive into the fundamental principles of atomic structure, quantum mechanics, and the periodic table. The f subshell's importance extends beyond its electron capacity; it shapes the properties of a significant portion of the periodic table, influencing diverse technologies and applications. The intricacies of electron configuration and the subtle deviations from predicted filling orders showcase the complex and fascinating world of quantum mechanics and its impact on the macroscopic world. The seemingly simple question opens a window into the elegant and intricate design of matter at the atomic level.
Latest Posts
Latest Posts
-
How Do You Separate Sugar And Water
Apr 09, 2025
-
Which Of The Following Is An Application Of Conservatism
Apr 09, 2025
-
Long Chains Of Amino Acids Joined Together By Peptide Bonds
Apr 09, 2025
-
Which Of The Following Is Not A White Blood Cell
Apr 09, 2025
-
C6h12o6 6o2 6co2 6h2o Energy
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons In F Subshell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.