What Is The Most Reactive Metal On The Periodic Table
News Leon
Mar 26, 2025 · 4 min read
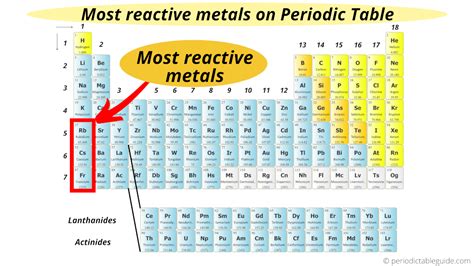
Table of Contents
What is the Most Reactive Metal on the Periodic Table?
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One of the most striking properties differentiating elements is their reactivity, particularly concerning metals. While many metals exhibit reactivity, some stand out significantly. This article delves deep into the question: what is the most reactive metal on the periodic table? and explores the reasons behind its extreme reactivity. We will also examine related concepts and dispel some common misconceptions.
Understanding Reactivity
Before identifying the most reactive metal, let's define what we mean by "reactivity." In chemistry, reactivity refers to the tendency of a substance to undergo a chemical reaction. For metals, this usually involves the loss of electrons to form positive ions (cations). The more readily a metal loses electrons, the more reactive it is. This electron loss is driven by the metal's electronic configuration and its electronegativity – the ability to attract electrons. Metals with low electronegativity readily give up their electrons, making them highly reactive.
Factors Influencing Metal Reactivity
Several factors govern a metal's reactivity:
-
Ionization Energy: This is the energy required to remove an electron from a neutral atom. Lower ionization energy indicates that electrons are more easily lost, leading to higher reactivity.
-
Electron Configuration: Metals with loosely held valence electrons (electrons in the outermost shell) tend to be more reactive because these electrons are more easily lost. Elements in Group 1 (alkali metals) and Group 2 (alkaline earth metals) exemplify this.
-
Atomic Radius: A larger atomic radius means the outermost electrons are farther from the nucleus and experience less attraction, making them easier to remove and increasing reactivity.
-
Electropositivity: This refers to a metal's tendency to lose electrons and form positive ions. Highly electropositive metals are highly reactive.
The Contenders: Alkali Metals
The alkali metals (Group 1 elements: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), and Francium (Fr)) are renowned for their exceptional reactivity. Their single valence electron is loosely held, making them eager electron donors. As you move down the group, the atomic radius increases, and the ionization energy decreases, leading to a progressive increase in reactivity.
Why Alkali Metals are So Reactive
The alkali metals' reactivity stems from their electronic configuration. Their outermost shell contains only one electron, which is relatively far from the nucleus and experiences weak attraction. This lone electron is easily lost to achieve a stable electron configuration like that of a noble gas, making them highly reactive.
The Champion: Francium
Based on the trends within the alkali metals, Francium (Fr) is considered the most reactive metal. This highly radioactive element sits at the bottom of Group 1. Its large atomic radius and extremely low ionization energy allow it to lose its valence electron exceptionally easily. This exceptionally high reactivity means Francium reacts violently with water and even with air, making its study extremely challenging.
The Challenges of Studying Francium
Francium's extreme radioactivity presents significant challenges to its study. Its short half-life (only 22 minutes for the most stable isotope) makes it incredibly difficult to obtain and handle in sufficient quantities for extensive experimentation. Its reactivity is so intense that even minute amounts react explosively with water, releasing substantial energy and hydrogen gas. This limits our direct experimental data on its exact reactivity compared to other alkali metals.
Dispelling Misconceptions
Some misconceptions surround the most reactive metal:
-
Cesium is sometimes cited as the most reactive: While Cesium is indeed extremely reactive, Francium's larger atomic size and even lower ionization energy give it the edge in reactivity, theoretically.
-
Reactivity is only about reaction speed: While reaction speed is a key aspect, reactivity is a broader concept encompassing the tendency to react and the energy released during the reaction.
Beyond Reactivity: Other Important Properties
While reactivity is a crucial property, it's essential to understand that other factors contribute to a metal's overall usefulness and applications. These include:
-
Density: Francium's high density contributes to its unique physical properties.
-
Melting and Boiling Points: The comparatively low melting and boiling points of Francium are important considerations.
-
Radioactivity: Francium's inherent radioactivity restricts its use in practical applications, despite its extremely high reactivity.
Conclusion: The Reigning Champion and its Limitations
In summary, although definitive experimental proof is limited due to Francium’s radioactivity and scarcity, Francium (Fr) is widely considered the most reactive metal on the periodic table. Its unique electronic configuration, large atomic radius, and exceptionally low ionization energy all contribute to this extreme reactivity. However, its radioactivity and short half-life restrict its practical applications and hamper detailed experimental analysis. The study of Francium's reactivity remains a fascinating frontier in chemistry, highlighting both the predictive power of the periodic table and the challenges of studying highly unstable elements. Further research may refine our understanding of Francium's properties and perhaps uncover even more reactive, yet-to-be-discovered elements in the future.
Latest Posts
Latest Posts
-
Why Is It Important That The Cell Membrane Is Flexible
Mar 29, 2025
-
What Is The Role Of Nad In Cellular Respiration
Mar 29, 2025
-
The Simplest Rationalising Factor Of Root 50
Mar 29, 2025
-
A Particle Moves Along A Straight Line
Mar 29, 2025
-
During Meiosis Homologous Chromosomes Separate At
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Most Reactive Metal On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
