What Is The Molarity Of Water
News Leon
Mar 15, 2025 · 5 min read
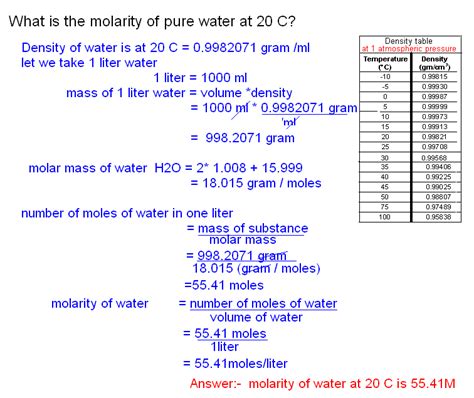
Table of Contents
What is the Molarity of Water? A Deep Dive into Water's Concentration
The seemingly simple question, "What is the molarity of water?", opens a door to a fascinating exploration of chemistry, concentration calculations, and the unique properties of water. While the answer itself might seem straightforward, understanding the underlying concepts and calculations requires a deeper dive into molarity and the characteristics of water. This comprehensive guide will explore the molarity of water, examining the definition of molarity, the calculations involved, and the implications of water's unique molarity.
Understanding Molarity: A Foundation for Calculation
Before we delve into the molarity of water, let's establish a firm understanding of the concept of molarity itself. Molarity (M), also known as molar concentration, is a measure of the concentration of a solute in a solution. It's defined as the number of moles of solute per liter of solution. The formula is elegantly simple:
Molarity (M) = Moles of solute / Liters of solution
This means that a 1 Molar (1M) solution contains one mole of solute dissolved in one liter of solution. It's crucial to remember that the volume refers to the total volume of the solution, not just the volume of the solvent.
Calculating the Molar Mass of Water
To determine the molarity of water, we first need to calculate its molar mass. Water (H₂O) is composed of two hydrogen atoms and one oxygen atom. Using the atomic masses from the periodic table (approximately 1 g/mol for hydrogen and 16 g/mol for oxygen), we calculate the molar mass of water as follows:
- Hydrogen (H): 2 atoms * 1 g/mol/atom = 2 g/mol
- Oxygen (O): 1 atom * 16 g/mol/atom = 16 g/mol
- Total Molar Mass (H₂O): 2 g/mol + 16 g/mol = 18 g/mol
This means that one mole of water has a mass of 18 grams.
Determining the Density of Water
The density of water is another critical factor in calculating its molarity. While the density of water varies slightly with temperature and pressure, we often use the standard value of approximately 1 gram per milliliter (g/mL) or 1 kilogram per liter (kg/L) at 4°C. This means that one liter of water weighs approximately one kilogram (1000 grams).
Calculating the Molarity of Pure Water
Now we can combine the molar mass and density to calculate the molarity of pure water. We know that:
- One liter of water weighs approximately 1000 grams.
- One mole of water weighs 18 grams.
Therefore, the number of moles in one liter of water is:
Moles of water = (Mass of water) / (Molar mass of water) = 1000 g / 18 g/mol ≈ 55.56 moles
Using the molarity formula:
Molarity of water = Moles of water / Liters of water = 55.56 moles / 1 L ≈ 55.56 M
Therefore, the molarity of pure water is approximately 55.56 M.
The Significance of Water's High Molarity
The high molarity of water (55.56 M) is not just a numerical value; it has significant implications in various chemical and biological processes. This high concentration of water molecules directly influences:
1. Solvent Properties:
Water's high molarity contributes to its exceptional solvent properties. The abundance of water molecules facilitates the dissolution of many polar and ionic substances, making it a crucial medium for biological reactions and chemical processes.
2. Chemical Reactions:
Many chemical reactions, particularly in biological systems, occur in aqueous solutions. Water's high molarity influences reaction rates and equilibrium constants. The sheer number of water molecules participating in the solution plays a substantial role.
3. Hydrogen Bonding:
Water's high concentration influences the prevalence of hydrogen bonding. The extensive hydrogen bonding network in water is responsible for its unique properties like high surface tension, high boiling point, and high specific heat capacity. These properties underpin many biological processes.
4. Biological Processes:
In living organisms, water acts as a solvent, a reactant, and a product in various metabolic pathways. Its high molarity is essential for maintaining cellular structure, transport of nutrients, and the regulation of biological functions.
Molarity vs. Molality: Understanding the Difference
It's important to differentiate between molarity and molality. While molarity is defined as moles of solute per liter of solution, molality (m) is defined as moles of solute per kilogram of solvent. In the case of pure water, the distinction is less pronounced because the density of water is close to 1 g/mL. However, for solutions with significant solute concentrations, the difference between molarity and molality becomes more pronounced.
Applications and Implications: Exploring Real-World Scenarios
The concept of molarity, especially in relation to water, has practical applications in diverse fields:
1. Analytical Chemistry:
Molarity is crucial in analytical chemistry for preparing solutions of known concentrations. Accurate molarity calculations are essential for quantitative analysis.
2. Biochemistry and Molecular Biology:
In biochemistry and molecular biology, molarity is critical in determining the concentrations of various molecules, such as proteins, enzymes, and nucleic acids. Accurate concentration measurements are vital for experiments and analyses.
3. Environmental Science:
Water quality analysis often involves determining the molarity of various dissolved substances in water samples. This is crucial for assessing the health and pollution levels of water bodies.
4. Industrial Processes:
Many industrial processes rely on precise control of reactant concentrations. Accurate molarity calculations ensure the efficient and safe operation of chemical plants and manufacturing facilities.
Conclusion: The Undeniable Importance of Water's Molarity
The seemingly simple calculation of the molarity of water reveals a deeper understanding of the fundamental concepts of chemistry and its relevance to diverse fields. The high molarity of water, approximately 55.56 M, is not merely a number; it's a critical parameter influencing numerous physical, chemical, and biological processes. Understanding this concentration helps us appreciate the unique properties of water and its indispensable role in our world. From its solvent properties to its involvement in chemical reactions and its crucial role in biological systems, the high molarity of water underlies the fundamental processes that shape our environment and sustains life itself. Mastering the concept of molarity and applying it to water allows for a deeper appreciation of the intricate balance of nature and the scientific principles that govern it.
Latest Posts
Latest Posts
-
Difference Between Interest Groups And Political Parties
Mar 15, 2025
-
The Division Of The Cell Nucleus Is Called
Mar 15, 2025
-
The Summer Of The White Beautiful Horse
Mar 15, 2025
-
Which Phase Of Cell Cycle Is Longest
Mar 15, 2025
-
Structure From Which Chordae Tendineae Originate
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molarity Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
