What Is The Molar Mass Of Sucrose
News Leon
Mar 15, 2025 · 5 min read
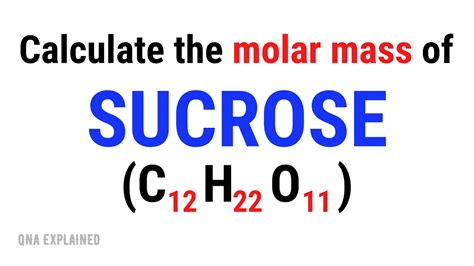
Table of Contents
What is the Molar Mass of Sucrose? A Deep Dive into Molecular Weight Calculation
Sucrose, also known as table sugar, is a disaccharide composed of glucose and fructose. Understanding its molar mass is crucial in various fields, from chemistry and food science to medicine and biochemistry. This comprehensive guide will delve into the calculation of sucrose's molar mass, explore its significance, and discuss related concepts.
Understanding Molar Mass
Molar mass, also known as molecular weight, represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as 6.022 x 10²³ (Avogadro's number) of entities, whether they are atoms, molecules, or ions. The molar mass is expressed in grams per mole (g/mol). Knowing the molar mass allows us to accurately convert between mass and the number of moles of a substance, which is essential for stoichiometric calculations in chemical reactions.
Calculating the Molar Mass of Sucrose (C₁₂H₂₂O₁₁)
Sucrose has the chemical formula C₁₂H₂₂O₁₁. To calculate its molar mass, we need the atomic masses of carbon (C), hydrogen (H), and oxygen (O). These values can be found on the periodic table.
- Carbon (C): Approximately 12.01 g/mol
- Hydrogen (H): Approximately 1.01 g/mol
- Oxygen (O): Approximately 16.00 g/mol
Now, let's calculate the molar mass of sucrose:
- Carbon (C): 12 atoms of carbon x 12.01 g/mol/atom = 144.12 g/mol
- Hydrogen (H): 22 atoms of hydrogen x 1.01 g/mol/atom = 22.22 g/mol
- Oxygen (O): 11 atoms of oxygen x 16.00 g/mol/atom = 176.00 g/mol
Total Molar Mass: 144.12 g/mol + 22.22 g/mol + 176.00 g/mol = 342.34 g/mol
Therefore, the molar mass of sucrose is approximately 342.34 g/mol. Slight variations may occur depending on the atomic mass values used, as these are typically rounded for convenience.
The Significance of Sucrose's Molar Mass
Knowing the molar mass of sucrose is vital in various applications:
1. Stoichiometry and Chemical Reactions
In chemical reactions involving sucrose, its molar mass is crucial for determining the amounts of reactants and products. For instance, if we want to calculate how much carbon dioxide is produced from the complete combustion of a certain mass of sucrose, we need its molar mass to convert the mass of sucrose into moles, allowing us to use the stoichiometric ratios from the balanced chemical equation.
2. Food Science and Nutrition
The molar mass is used in food science to determine the precise amounts of sucrose in various food products. This information is crucial for nutritional labeling and understanding the caloric content of foods, as sucrose contributes significantly to the overall energy value. Calculations involving molarity (moles per liter) are also relevant for determining sucrose concentrations in solutions used in food processing.
3. Medicine and Biochemistry
Sucrose plays a role in several medical applications, including intravenous solutions and as a component in certain pharmaceuticals. Its molar mass is essential for accurately preparing solutions with specific concentrations, ensuring the correct dosage and avoiding adverse reactions. In biochemistry, understanding the molar mass is essential for studying sucrose metabolism and its interactions with other molecules in biological systems.
4. Industrial Applications
Sucrose is widely used in various industries, including confectionery, brewing, and textiles. Precise calculations involving its molar mass are necessary for controlling the properties of the final products and optimizing production processes. For example, understanding the molar mass helps determine the appropriate amount of sucrose to achieve a desired sweetness or viscosity in a product.
Related Concepts and Calculations
Several related concepts are interconnected with the molar mass of sucrose:
1. Molecular Formula and Empirical Formula
The molecular formula (C₁₂H₂₂O₁₁) provides the exact number of atoms of each element in a molecule of sucrose. The empirical formula, on the other hand, represents the simplest whole-number ratio of atoms in a compound. In the case of sucrose, the molecular formula and empirical formula are the same because the numbers of atoms are already in their simplest whole-number ratio.
2. Molarity and Molality
Molarity (M) is the number of moles of solute per liter of solution, while molality (m) is the number of moles of solute per kilogram of solvent. Knowing the molar mass of sucrose is crucial for calculating both molarity and molality when preparing sucrose solutions.
3. Mass Percentage
The mass percentage of an element in a compound represents the percentage of the element's mass in the total mass of the compound. The molar mass of sucrose can be used to calculate the mass percentage of carbon, hydrogen, and oxygen in sucrose.
4. Avogadro's Number and the Mole Concept
Avogadro's number (6.022 x 10²³) is the cornerstone of the mole concept. It establishes the relationship between the number of particles (atoms, molecules) and the amount of substance in moles. The molar mass directly ties into this concept, as it represents the mass of Avogadro's number of molecules of a substance.
Practical Applications and Examples
Let's look at some practical examples demonstrating the use of sucrose's molar mass:
Example 1: Preparing a Sucrose Solution
Suppose we need to prepare 1 liter of a 0.5 M sucrose solution. We can use the molar mass to determine the required mass of sucrose:
- Moles of sucrose needed: 0.5 mol/L x 1 L = 0.5 mol
- Mass of sucrose needed: 0.5 mol x 342.34 g/mol = 171.17 g
Therefore, we need to dissolve 171.17 g of sucrose in enough water to make 1 liter of solution.
Example 2: Calculating the Number of Molecules
If we have 100 g of sucrose, we can calculate the number of sucrose molecules:
- Moles of sucrose: 100 g / 342.34 g/mol = 0.292 mol
- Number of molecules: 0.292 mol x 6.022 x 10²³ molecules/mol = 1.76 x 10²³ molecules
Conclusion
The molar mass of sucrose (approximately 342.34 g/mol) is a fundamental value with far-reaching implications across various scientific and industrial disciplines. Understanding its calculation and significance is essential for accurate stoichiometric calculations, solution preparation, nutritional analysis, and numerous other applications. The concepts discussed in this article provide a solid foundation for further explorations in chemistry, biochemistry, and related fields. Mastering these calculations is key to success in many scientific endeavors. Remember to always double-check your calculations and use updated atomic mass values for the highest accuracy.
Latest Posts
Latest Posts
-
Difference Between Interest Groups And Political Parties
Mar 15, 2025
-
The Division Of The Cell Nucleus Is Called
Mar 15, 2025
-
The Summer Of The White Beautiful Horse
Mar 15, 2025
-
Which Phase Of Cell Cycle Is Longest
Mar 15, 2025
-
Structure From Which Chordae Tendineae Originate
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Sucrose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
