What Is The Molar Mass Of Ch3oh
News Leon
Mar 21, 2025 · 5 min read
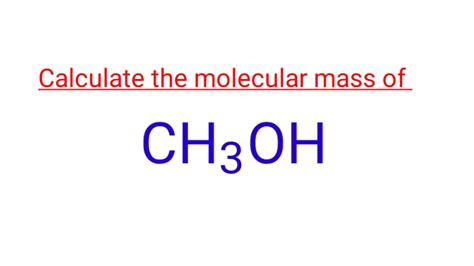
Table of Contents
What is the Molar Mass of CH3OH? A Comprehensive Guide
Determining the molar mass of a compound is a fundamental concept in chemistry. It's crucial for various calculations, including stoichiometry, determining solution concentrations, and understanding chemical reactions. This comprehensive guide will delve into the calculation of the molar mass of methanol (CH3OH), explaining the process step-by-step and exploring related concepts. We'll also discuss the significance of molar mass and its applications in various fields.
Understanding Molar Mass
Molar mass, also known as molecular weight, is the mass of one mole of a substance. A mole is a unit of measurement in chemistry that represents Avogadro's number (approximately 6.022 x 10²³) of particles, whether they are atoms, molecules, ions, or other specified entities. The molar mass is expressed in grams per mole (g/mol).
Calculating the Molar Mass of CH3OH (Methanol)
Methanol, with the chemical formula CH₃OH, is a simple organic compound, also known as methyl alcohol or wood alcohol. To calculate its molar mass, we need to consider the atomic masses of each element present in the molecule:
- Carbon (C): Approximately 12.01 g/mol
- Hydrogen (H): Approximately 1.01 g/mol
- Oxygen (O): Approximately 16.00 g/mol
Step-by-step Calculation:
-
Identify the number of atoms of each element: In CH₃OH, we have:
- 1 Carbon atom
- 4 Hydrogen atoms (3 from CH₃ and 1 from OH)
- 1 Oxygen atom
-
Multiply the number of atoms of each element by its atomic mass:
- Carbon: 1 atom * 12.01 g/mol = 12.01 g/mol
- Hydrogen: 4 atoms * 1.01 g/mol = 4.04 g/mol
- Oxygen: 1 atom * 16.00 g/mol = 16.00 g/mol
-
Sum the masses of all the atoms:
- Total molar mass = 12.01 g/mol + 4.04 g/mol + 16.00 g/mol = 32.05 g/mol
Therefore, the molar mass of CH₃OH (methanol) is approximately 32.05 g/mol.
Significance of Molar Mass
The molar mass of a compound is a critical piece of information in numerous chemical calculations and applications. Here are some key uses:
1. Stoichiometry:
Stoichiometry involves the quantitative relationships between reactants and products in chemical reactions. Knowing the molar mass allows us to convert between the mass of a substance and the number of moles, which is essential for balancing equations and predicting the amounts of products formed.
For example, if we know the mass of methanol reacted in a specific reaction, we can use its molar mass to determine the number of moles involved, allowing us to calculate the amount of product formed based on the stoichiometric ratios in the balanced chemical equation.
2. Solution Chemistry:
Molar mass is essential for preparing solutions of a specific concentration. Chemists frequently work with molarity (M), which is defined as the number of moles of solute per liter of solution. To prepare a solution of a particular molarity, you need to know the molar mass of the solute to calculate the required mass to dissolve in a given volume of solvent.
3. Gas Laws:
The ideal gas law (PV = nRT) relates pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). Molar mass plays a role in connecting the mass of a gas to its number of moles, allowing for calculations involving gas volume, pressure, and temperature.
4. Determining Empirical and Molecular Formulas:
Molar mass is crucial in determining the molecular formula of a compound. Often, experiments will provide the empirical formula (the simplest whole-number ratio of atoms in a compound). Knowing the molar mass of the compound allows us to determine the molecular formula, which represents the actual number of atoms of each element in a molecule.
5. Physical Properties Predictions:
While not a direct relationship, the molar mass often correlates with certain physical properties of a substance, such as boiling point and melting point. Higher molar mass generally indicates stronger intermolecular forces, leading to higher boiling and melting points. This is a useful generalization, although other factors also influence these properties.
Applications of Methanol and its Molar Mass Calculations
Understanding the molar mass of methanol is vital due to its widespread applications across various industries. Methanol's diverse uses include:
-
Fuel: Methanol is used as a fuel additive, particularly in racing cars, and is being explored as a potential alternative fuel source. Its molar mass is crucial in determining fuel efficiency and combustion calculations.
-
Solvent: Methanol is a versatile solvent used in many industrial processes and chemical reactions. Accurate molar mass calculations ensure precise control over the concentrations of solutions used in these processes.
-
Chemical Synthesis: Methanol serves as a building block for producing other chemicals, including formaldehyde, acetic acid, and methyl tert-butyl ether (MTBE). Molar mass calculations are essential for controlling the stoichiometry of these syntheses.
-
Biofuel Production: Methanol production from biomass is a promising avenue for renewable energy. Molar mass calculations are vital in optimizing the efficiency of these biofuel production processes.
Beyond the Basics: Isotopes and Atomic Mass
The atomic masses used in our calculation are average atomic masses, reflecting the natural abundance of different isotopes of each element. Isotopes are atoms of the same element with different numbers of neutrons. For instance, carbon has several isotopes, including ¹²C and ¹³C. The average atomic mass accounts for the proportion of each isotope present in nature. This means that the molar mass of CH₃OH we calculated is an average molar mass, representing a sample of methanol containing the naturally occurring isotopic abundances. In situations requiring higher precision, the specific isotopic composition of the methanol sample would need to be considered.
Conclusion:
Calculating the molar mass of CH₃OH, or any compound, is a fundamental skill in chemistry with far-reaching applications. The process involves adding the atomic masses of each atom present in the molecule. The accuracy of this calculation is vital for various applications, from stoichiometric calculations to solution preparation and beyond. Understanding the concepts of molar mass and its significance opens doors to a deeper comprehension of chemical principles and their practical applications in diverse fields. The seemingly simple calculation of molar mass is a cornerstone of chemical understanding and plays a pivotal role in numerous scientific endeavors.
Latest Posts
Latest Posts
-
Which Of The Following Are Prokaryotes
Mar 23, 2025
-
Increases To Owners Equity May Be From
Mar 23, 2025
-
Figure A Shows A Circular Disk That Is Uniformly Charged
Mar 23, 2025
-
One Organism Benefits And The Other Is Harmed
Mar 23, 2025
-
What Is The Size Of India
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Ch3oh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
