What Is The Molar Mass Of Bf3
News Leon
Mar 24, 2025 · 6 min read
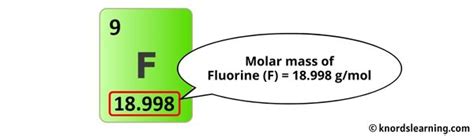
Table of Contents
What is the Molar Mass of BF3? A Comprehensive Guide
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. This article delves deep into calculating the molar mass of boron trifluoride (BF₃), explaining the process step-by-step and exploring its significance in different chemical contexts. We'll also touch upon related concepts like molecular weight and the applications of knowing the molar mass of BF₃.
Understanding Molar Mass
Before we calculate the molar mass of BF₃, let's clarify the concept. Molar mass is the mass of one mole of a substance. A mole is a unit of measurement in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). The molar mass is numerically equivalent to the molecular weight but expressed in grams per mole (g/mol). Molecular weight, on the other hand, is a dimensionless quantity, representing the sum of the atomic weights of all the atoms in a molecule.
Calculating the Molar Mass of BF₃
Boron trifluoride (BF₃) is a colorless, toxic gas with a pungent odor. To calculate its molar mass, we need the atomic masses of boron (B) and fluorine (F) from the periodic table.
- Atomic mass of Boron (B): Approximately 10.81 g/mol
- Atomic mass of Fluorine (F): Approximately 18.998 g/mol
BF₃ has one boron atom and three fluorine atoms. Therefore, the molar mass is calculated as follows:
Molar Mass (BF₃) = (1 × Atomic mass of B) + (3 × Atomic mass of F)
Molar Mass (BF₃) = (1 × 10.81 g/mol) + (3 × 18.998 g/mol)
Molar Mass (BF₃) = 10.81 g/mol + 56.994 g/mol
Molar Mass (BF₃) ≈ 67.81 g/mol
Therefore, the molar mass of boron trifluoride (BF₃) is approximately 67.81 grams per mole.
Significance of Knowing the Molar Mass of BF₃
Understanding the molar mass of BF₃ is essential for several reasons:
1. Stoichiometric Calculations:
Knowing the molar mass allows for accurate stoichiometric calculations. Stoichiometry is the relationship between the quantities of reactants and products in a chemical reaction. For instance, if you're reacting BF₃ with another compound, you'll need to know its molar mass to determine the correct mole ratios for a balanced reaction. This is crucial for predicting the yield of a reaction and optimizing experimental procedures.
2. Concentration Calculations:
Molar mass is fundamental when dealing with molarity (M), a unit of concentration expressing moles of solute per liter of solution. If you need to prepare a solution of BF₃ with a specific molarity, you'll need to calculate the mass of BF₃ required using its molar mass. This is essential in many chemical and biological experiments.
3. Determining Empirical and Molecular Formulas:
Molar mass plays a vital role in determining the empirical and molecular formulas of unknown compounds. Empirical formulas represent the simplest whole-number ratio of atoms in a compound, while molecular formulas represent the actual number of atoms in a molecule. Knowing the molar mass of an unknown compound alongside its percentage composition allows chemists to determine both the empirical and molecular formulas.
4. Gas Law Calculations:
The ideal gas law (PV = nRT) relates pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). The number of moles (n) can be calculated using the mass of the gas and its molar mass. Thus, the molar mass of BF₃ is critical for solving various gas law problems involving this compound.
5. Understanding Physical Properties:
Molar mass influences various physical properties, including density, boiling point, and melting point. By understanding the molar mass of BF₃, we can better predict its behavior under different conditions. This is crucial for safe handling and storage of this potentially hazardous compound.
Applications of BF₃
Boron trifluoride, despite its toxicity, finds numerous applications in various fields:
1. Catalyst in Organic Chemistry:
BF₃ is a powerful Lewis acid catalyst used extensively in organic chemistry. It facilitates various reactions, including Friedel-Crafts alkylations and acylations, as well as esterification reactions. Its ability to accept electron pairs makes it an effective catalyst for reactions involving electron-rich substrates. Understanding its molar mass is vital for precise control of reaction conditions and stoichiometry.
2. Doping Agent in Semiconductor Industry:
In the semiconductor industry, BF₃ is used as a doping agent in the production of silicon-based semiconductors. Doping involves introducing impurities into a semiconductor material to modify its electrical conductivity. BF₃ is used to introduce boron atoms into silicon, creating p-type semiconductors. Precise control of doping levels requires accurate knowledge of the molar mass of BF₃.
3. Synthesis of other Boron Compounds:
BF₃ serves as a precursor in the synthesis of other important boron compounds. Its reactivity allows for the creation of various boron-containing materials with specific properties. Accurate stoichiometric calculations, relying heavily on its molar mass, are essential for efficient and controlled synthesis.
Safety Precautions when Handling BF₃
It's crucial to emphasize that boron trifluoride is a highly toxic and corrosive gas. Direct contact with BF₃ can cause severe burns to the skin and eyes. Inhalation can lead to respiratory irritation and other serious health issues. Therefore, handling BF₃ requires stringent safety precautions, including:
- Working in a well-ventilated area or using a fume hood: To minimize exposure to the gas.
- Wearing appropriate personal protective equipment (PPE): Including gloves, goggles, and a respirator.
- Following proper handling and storage procedures: As outlined in safety data sheets (SDS).
Always prioritize safety when working with BF₃ or any other hazardous chemical.
Further Exploration
The molar mass of BF₃, while seemingly a simple calculation, is a cornerstone for understanding its chemical behavior and applications. This seemingly basic calculation opens doors to complex stoichiometric analysis, reaction optimization, and applications spanning diverse fields. The information provided here offers a solid foundation for further exploration into the fascinating world of boron chemistry and its industrial applications. Further research into the synthesis methods, applications, and environmental impacts of BF₃ will deepen your understanding of this important compound. Consider exploring advanced topics like the kinetics and thermodynamics of reactions involving BF₃, or delve deeper into the electronic structure of the molecule to better understand its reactivity. The accurate determination of its molar mass is not merely an academic exercise but a critical step in responsible scientific practice.
Conclusion
In conclusion, the molar mass of BF₃ is approximately 67.81 g/mol. This seemingly straightforward calculation holds immense significance, underpinning various aspects of chemistry, from stoichiometric calculations to industrial applications. Understanding this fundamental concept is critical for anyone working with this compound, be it in a laboratory setting or an industrial process. Remember always to prioritize safety when handling this hazardous material. This detailed explanation should provide a solid understanding of the importance and calculation of the molar mass of BF₃, as well as its wide-ranging applications and crucial safety considerations.
Latest Posts
Latest Posts
-
Round 64 To The Nearest Ten
Mar 26, 2025
-
Which Of The Following Are Meso Compounds
Mar 26, 2025
-
Words Beginning With The Same Letter
Mar 26, 2025
-
The Proper Order For The Scientific Process Is
Mar 26, 2025
-
What Is The Lightest Element On The Periodic Table
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Bf3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
