What Is The Molar Mass Of Aucl3
News Leon
Mar 17, 2025 · 5 min read
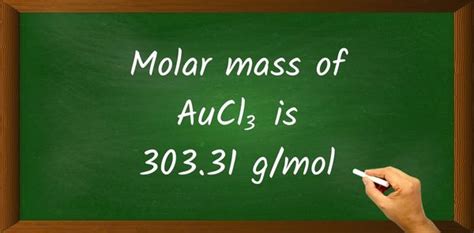
Table of Contents
What is the Molar Mass of AuCl₃? A Deep Dive into Gold(III) Chloride
Gold(III) chloride, or AuCl₃, is a fascinating compound with a rich history and important applications in various fields. Understanding its properties, including its molar mass, is crucial for anyone working with this substance in chemistry, materials science, or related disciplines. This comprehensive article will delve into the calculation of AuCl₃'s molar mass, explore its properties, and discuss its significance.
Understanding Molar Mass
Before we calculate the molar mass of AuCl₃, let's refresh our understanding of this fundamental concept. Molar mass is the mass of one mole of a substance. A mole is a unit of measurement in chemistry that represents Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). The molar mass is expressed in grams per mole (g/mol). To determine the molar mass of a compound, we need to consider the molar mass of each element present in the compound and the number of atoms of each element.
Calculating the Molar Mass of AuCl₃
Gold(III) chloride, AuCl₃, consists of one gold (Au) atom and three chlorine (Cl) atoms. To calculate its molar mass, we need the atomic masses of gold and chlorine. These values can be found on the periodic table of elements.
- Atomic mass of Gold (Au): Approximately 196.97 g/mol
- Atomic mass of Chlorine (Cl): Approximately 35.45 g/mol
Now, let's perform the calculation:
Molar mass of AuCl₃ = (1 × Atomic mass of Au) + (3 × Atomic mass of Cl)
Molar mass of AuCl₃ = (1 × 196.97 g/mol) + (3 × 35.45 g/mol)
Molar mass of AuCl₃ = 196.97 g/mol + 106.35 g/mol
Molar mass of AuCl₃ = 303.32 g/mol
Therefore, the molar mass of AuCl₃ is approximately 303.32 g/mol. This value is crucial for various stoichiometric calculations involving AuCl₃, such as determining the amount of reactant needed in a chemical reaction or the yield of a product.
Properties of AuCl₃
Gold(III) chloride is a reddish-brown crystalline solid. It's highly reactive and readily dissolves in water, forming a yellow solution. Its properties contribute to its diverse applications.
Chemical Properties:
- Reactivity: AuCl₃ is a strong oxidizing agent, meaning it readily accepts electrons from other substances. This property makes it useful in various redox reactions.
- Solubility: Its solubility in water and other solvents is key to its use in various chemical processes.
- Complex Formation: AuCl₃ readily forms complexes with various ligands, expanding its applications in coordination chemistry.
Physical Properties:
- Appearance: Reddish-brown crystalline solid
- Melting Point: Relatively low melting point compared to other gold compounds.
- Solubility: Soluble in water, ethanol, and other polar solvents.
Applications of AuCl₃
The unique properties of AuCl₃ make it valuable in several applications:
1. Catalysis:
AuCl₃ acts as a catalyst in various organic reactions. Its ability to facilitate chemical transformations without being consumed makes it essential in several industrial processes.
2. Electroplating:
In the electroplating industry, AuCl₃ is used to deposit a thin layer of gold onto other materials, enhancing their appearance, durability, and conductivity. This application is crucial in jewelry making, electronics, and other fields.
3. Medicine and Pharmaceuticals:
Although less common, AuCl₃ has seen some exploration in medicine. Some gold compounds have anti-inflammatory properties, and AuCl₃ has been investigated for its potential role in treating certain medical conditions, though its use is limited due to toxicity concerns.
4. Photography:
Historically, AuCl₃ has had some applications in photography, primarily in toning photographic prints to enhance their appearance and stability.
5. Synthesis of Gold Nanoparticles:
AuCl₃ is a common precursor in the synthesis of gold nanoparticles. These nanoparticles possess unique optical, electronic, and catalytic properties, making them crucial in various nanotechnology applications. This includes biosensors, drug delivery systems, and catalysts.
Safety Precautions when Handling AuCl₃
Gold(III) chloride is a corrosive substance, and handling it requires appropriate safety measures:
- Personal Protective Equipment (PPE): Always wear appropriate PPE, including gloves, eye protection, and a lab coat, when handling AuCl₃.
- Ventilation: Work in a well-ventilated area or use a fume hood to prevent inhalation of any dust or fumes.
- Disposal: Dispose of AuCl₃ and its waste according to local regulations and guidelines.
- Storage: Store AuCl₃ in a tightly sealed container in a cool, dry place, away from incompatible materials.
Further Considerations and Related Compounds
While AuCl₃ is the focus of this article, it's important to note its relationship to other gold compounds. Gold can exhibit different oxidation states, leading to compounds like AuCl (gold(I) chloride) which possesses different properties and applications. Understanding the chemistry of gold in different oxidation states is crucial for a comprehensive understanding of its diverse applications.
The molar mass calculation, while seemingly straightforward, is essential for accurate stoichiometric calculations. Errors in determining the molar mass can lead to significant inaccuracies in experimental results and industrial processes.
The applications of AuCl₃ are constantly evolving, with ongoing research exploring new and innovative uses in fields such as nanotechnology and catalysis. This highlights the importance of understanding the fundamental properties of this compound and its role in scientific and technological advancements.
Conclusion
The molar mass of AuCl₃ is precisely calculated as 303.32 g/mol. This simple yet crucial value underpins many calculations in chemistry, allowing researchers and professionals to accurately quantify reactants, products, and yields in reactions involving this valuable compound. Beyond the molar mass, the unique chemical and physical properties of AuCl₃ underpin its diverse applications in catalysis, electroplating, and the burgeoning field of nanotechnology, emphasizing its importance in various scientific and industrial settings. Remember to always prioritize safety when handling this compound. Further research and development continue to unveil new and exciting applications for AuCl₃, cementing its position as a vital compound in the world of chemistry and beyond.
Latest Posts
Latest Posts
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
-
How Many Water Molecules In A Drop Of Water
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Aucl3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
