What Is The Molar Mass Of Al No3 3
News Leon
Mar 31, 2025 · 5 min read
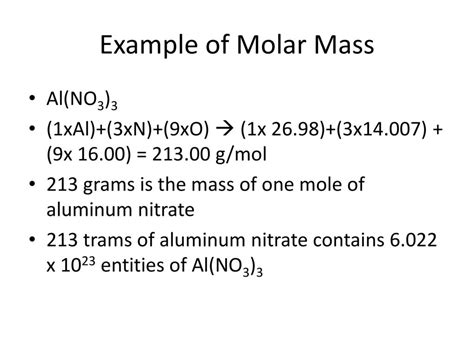
Table of Contents
What is the Molar Mass of Al(NO₃)₃? A Comprehensive Guide
Determining the molar mass of a compound like aluminum nitrate, Al(NO₃)₃, is a fundamental concept in chemistry. Understanding this calculation is crucial for various applications, from stoichiometry in chemical reactions to preparing solutions with specific concentrations. This comprehensive guide will walk you through the process of calculating the molar mass of Al(NO₃)₃, explain the underlying principles, and explore its significance in chemical calculations.
Understanding Molar Mass
Molar mass is defined as the mass of one mole of a substance. A mole is a unit of measurement in chemistry that represents Avogadro's number (approximately 6.022 x 10²³) of particles, whether they are atoms, molecules, ions, or formula units. The molar mass is numerically equal to the average atomic mass of an element or the sum of the average atomic masses of the atoms in a compound, expressed in grams per mole (g/mol).
Calculating the Molar Mass of Al(NO₃)₃
To calculate the molar mass of aluminum nitrate, Al(NO₃)₃, we need to consider the atomic masses of each element present in the compound: aluminum (Al), nitrogen (N), and oxygen (O). You can find these atomic masses on the periodic table.
1. Identify the elements and their atomic masses:
- Aluminum (Al): The atomic mass of aluminum is approximately 26.98 g/mol.
- Nitrogen (N): The atomic mass of nitrogen is approximately 14.01 g/mol.
- Oxygen (O): The atomic mass of oxygen is approximately 16.00 g/mol.
2. Determine the number of atoms of each element in the formula:
The chemical formula Al(NO₃)₃ tells us that one molecule of aluminum nitrate contains:
- 1 aluminum atom (Al)
- 3 nitrogen atoms (N)
- 9 oxygen atoms (O) (3 nitrate ions, each containing 3 oxygen atoms)
3. Calculate the molar mass:
Now, we can calculate the molar mass of Al(NO₃)₃ by multiplying the number of atoms of each element by its atomic mass and summing the results:
Molar mass of Al(NO₃)₃ = (1 × atomic mass of Al) + (3 × atomic mass of N) + (9 × atomic mass of O)
Molar mass of Al(NO₃)₃ = (1 × 26.98 g/mol) + (3 × 14.01 g/mol) + (9 × 16.00 g/mol)
Molar mass of Al(NO₃)₃ = 26.98 g/mol + 42.03 g/mol + 144.00 g/mol
Molar mass of Al(NO₃)₃ = 213.01 g/mol
Therefore, the molar mass of aluminum nitrate, Al(NO₃)₃, is approximately 213.01 g/mol.
Significance of Molar Mass in Chemical Calculations
The molar mass of Al(NO₃)₃, and other compounds, plays a vital role in various chemical calculations:
1. Stoichiometry:
Stoichiometry involves using balanced chemical equations to determine the quantitative relationships between reactants and products in a chemical reaction. Molar mass is essential for converting between mass and moles of substances, allowing us to determine the amount of reactants needed or products formed in a reaction. For example, if you know the mass of Al(NO₃)₃ reacting, you can use its molar mass to calculate the number of moles involved, and subsequently, the moles and mass of other reactants or products.
2. Solution Preparation:
Molar mass is critical for preparing solutions of specific concentrations. Chemists often work with molarity (M), which is defined as moles of solute per liter of solution. To prepare a solution of a certain molarity, you need to know the molar mass of the solute (like Al(NO₃)₃) to calculate the mass of solute needed to dissolve in a given volume of solvent.
3. Determining Empirical and Molecular Formulas:
Molar mass is essential in determining the empirical and molecular formulas of compounds. The empirical formula represents the simplest whole-number ratio of atoms in a compound. The molecular formula shows the actual number of atoms of each element in a molecule. Knowing the molar mass helps in converting the empirical formula to the molecular formula.
4. Gas Laws:
For gaseous compounds, molar mass is connected to the ideal gas law (PV = nRT). The molar mass allows for the conversion of gas volume to the number of moles, which is useful in various gas calculations, such as determining the density of a gas.
Potential Sources of Error in Molar Mass Calculation
While the calculation itself is relatively straightforward, several factors can contribute to slight variations in the calculated molar mass:
-
Isotopic Abundance: The atomic masses used in the calculation are average atomic masses, representing the weighted average of the masses of all isotopes of an element. The actual mass of an aluminum nitrate molecule will slightly vary depending on the specific isotopes present.
-
Rounding Errors: Rounding off atomic masses during the calculation can lead to minor inaccuracies in the final result. Using more significant figures in the atomic masses will improve accuracy.
-
Impurities: If the aluminum nitrate sample is impure, containing other substances, the calculated molar mass will be affected, and the value obtained will not represent the pure compound's molar mass.
Practical Applications of Aluminum Nitrate
Understanding the molar mass of Al(NO₃)₃ is crucial because aluminum nitrate is used in a wide range of applications, including:
-
Water Treatment: It's used as a coagulant in water treatment plants to remove impurities. Accurate molar mass calculations are vital for determining the appropriate dosage for effective water purification.
-
Textile Industry: Used as a mordant in dyeing processes to help fix the dye to the fabric.
-
Chemical Synthesis: Serves as a starting material or reagent in various chemical reactions. Precise molar mass calculations ensure the correct stoichiometric ratios are used in these reactions.
-
Catalysts: Can act as a catalyst in certain reactions.
-
Agriculture: Certain formulations are used as fertilizers and soil conditioners.
Conclusion
The molar mass of Al(NO₃)₃ is a crucial piece of information in many chemical calculations. Understanding how to calculate it, its significance in different contexts, and potential sources of error allows for accurate and reliable results in various chemical applications. The calculated molar mass of 213.01 g/mol serves as a fundamental parameter for working with aluminum nitrate in diverse scientific and industrial processes. Accurate determination of molar mass is not just a theoretical exercise; it’s a vital tool for practical applications and research in chemistry. Remember to always refer to a reliable periodic table for the most up-to-date atomic mass values to ensure accurate calculations.
Latest Posts
Latest Posts
-
The Way Of The World Synopsis
Apr 02, 2025
-
Ba No3 2 Na2so4 Balanced Equation
Apr 02, 2025
-
Which Phase Of Meiosis Separates Homologous Chromosomes
Apr 02, 2025
-
When Both Demand And Supply Change
Apr 02, 2025
-
Which Of The Following Statements Most Correctly Defines Homeostasis
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Al No3 3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
