What Is The Iupac Name Of The Compound Shown Below
News Leon
Mar 15, 2025 · 6 min read
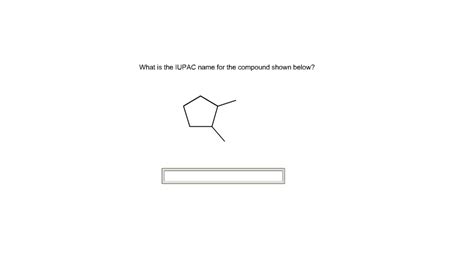
Table of Contents
What is the IUPAC Name of the Compound Shown Below? A Deep Dive into Organic Nomenclature
This article will explore the systematic naming of organic compounds according to IUPAC (International Union of Pure and Applied Chemistry) rules. We will delve into a detailed explanation of how to name complex organic molecules, focusing on identifying functional groups, parent chains, substituents, and applying the correct nomenclature prefixes and suffixes. While a specific compound isn't provided in the prompt, we will tackle several examples of increasing complexity, building the skills needed to name any organic molecule confidently.
Understanding the Basics of IUPAC Nomenclature
Before we tackle complex molecules, let's establish a strong foundation. IUPAC nomenclature is a systematic approach to naming organic compounds, ensuring a universal and unambiguous way to identify them. The system is based on several key principles:
-
Identifying the Parent Chain: This is the longest continuous carbon chain in the molecule. This chain forms the base name of the compound.
-
Identifying Functional Groups: These are atoms or groups of atoms that impart specific chemical properties to the molecule. Examples include alcohols (-OH), ketones (=O), carboxylic acids (-COOH), and amines (-NH₂). The presence of a functional group significantly influences the suffix of the IUPAC name.
-
Identifying Substituents: These are atoms or groups of atoms attached to the parent chain that are not part of the principal functional group. They are named as prefixes.
-
Numbering the Carbon Chain: The parent chain is numbered to assign the lowest possible numbers to substituents and the principal functional group.
-
Using Prefixes and Suffixes: Specific prefixes and suffixes are used to denote the number of carbon atoms in the parent chain, the types of substituents, and the principal functional group.
Alkane Nomenclature: The Foundation
Alkanes are the simplest organic compounds, consisting only of carbon and hydrogen atoms with single bonds. Their names form the basis for naming more complex molecules. The first ten alkanes and their IUPAC names are:
- Methane (CH₄)
- Ethane (C₂H₆)
- Propane (C₃H₈)
- Butane (C₄H₁₀)
- Pentane (C₅H₁₂)
- Hexane (C₆H₁₄)
- Heptane (C₇H₁₆)
- Octane (C₈H₁₈)
- Nonane (C₉H₂₀)
- Decane (C₁₀H₂₂)
For alkanes with more than ten carbons, Greek numerical prefixes are used (e.g., undecane, dodecane, etc.).
Introducing Substituents: Alkyl Groups
When hydrogen atoms on an alkane are replaced with other atoms or groups, we have substituents. Alkyl groups are derived from alkanes by removing one hydrogen atom. They are named by replacing the "-ane" suffix with "-yl." For example:
- Methyl (CH₃-)
- Ethyl (CH₃CH₂-)
- Propyl (CH₃CH₂CH₂-)
- Isopropyl (CH₃CH(CH₃)-)
- Butyl (various isomers exist)
Naming Branched Alkanes
Let's consider a branched alkane, for example, 2-methylbutane:
(Image of 2-methylbutane would be inserted here)
Step-by-step naming:
-
Identify the longest continuous carbon chain: This is a four-carbon chain (butane).
-
Identify the substituents: There is one methyl group (CH₃-) attached to the second carbon atom.
-
Number the carbon chain: Numbering from the end closest to the substituent gives the lowest number.
-
Write the name: The name is 2-methylbutane. The number indicates the position of the methyl group, and "butane" indicates the four-carbon parent chain.
Incorporating Functional Groups: Alcohols, Ketones, and Carboxylic Acids
The presence of functional groups significantly alters the naming process. The functional group determines the suffix of the IUPAC name.
Alcohols (-OH): The suffix "-ol" is added to the alkane name. The position of the hydroxyl group (-OH) is indicated by a number. For example, CH₃CH₂CH₂OH is propan-1-ol.
Ketones (=O): The suffix "-one" is added. The position of the carbonyl group (=O) is indicated by a number. For instance, CH₃COCH₃ is propan-2-one (also known as acetone).
Carboxylic Acids (-COOH): The suffix "-oic acid" is added. For example, CH₃COOH is ethanoic acid (also known as acetic acid).
Compounds with Multiple Functional Groups and Substituents: A Complex Example
Let's consider a more complex molecule:
(Image of a complex molecule with multiple substituents and functional groups would be inserted here. An example could be a molecule with a branched chain, an alcohol group, and a halogen substituent.)
Systematic Naming Process:
-
Identify the parent chain: Determine the longest continuous carbon chain containing the principal functional group (the one with the highest priority according to IUPAC rules).
-
Identify the principal functional group: This determines the suffix. The order of priority generally follows carboxylic acids > aldehydes > ketones > alcohols > amines, etc.
-
Identify and number substituents: Number the parent chain to give the lowest numbers to both the principal functional group and the substituents.
-
Name the substituents: Use the appropriate alkyl group names or other substituent names (e.g., chloro-, bromo-, etc.).
-
Combine the names: List the substituents alphabetically (ignoring prefixes like di-, tri-, etc.), followed by the numbered position of each substituent, and finally the name of the parent chain with the appropriate suffix. Numbers are separated from words by hyphens.
Dealing with Multiple Substituents: Prefixes and Alphabetical Ordering
When multiple identical substituents are present, prefixes like di-, tri-, tetra-, etc., are used. For instance, CH₃CH(CH₃)CH₃ is 2-methylpropane, while CH₃CH(CH₃)CH(CH₃)₂ is 2,3-dimethylbutane. The substituents are listed alphabetically, irrespective of their position numbers.
Stereoisomerism and its Impact on IUPAC Naming
Stereoisomerism, the existence of molecules with the same connectivity but different spatial arrangements, requires additional information in the IUPAC name. This includes specifying the configuration at chiral centers (using R/S descriptors) and the geometry of double bonds (using E/Z descriptors). These are crucial details to differentiate between stereoisomers, which can have significantly different properties.
Conclusion: Mastering IUPAC Nomenclature
Mastering IUPAC nomenclature is crucial for any student or professional working in chemistry. The system, though initially complex, provides a systematic and unambiguous way to name organic compounds, irrespective of their complexity. By understanding the fundamental principles discussed in this article—identifying the parent chain, functional groups, and substituents, and applying the correct prefixes and suffixes—one can systematically name a wide range of organic molecules. Consistent practice and familiarity with the rules are essential to develop proficiency. Remember to refer to the official IUPAC recommendations for the most accurate and updated guidelines. This in-depth exploration provides a strong foundation for confidently navigating the intricacies of organic chemical nomenclature and accurately identifying any given compound. Consistent practice and application of these rules will lead to mastery of this critical skill.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 4 And 9
Mar 15, 2025
-
Iodine Is Essential For The Synthesis Of
Mar 15, 2025
-
Is Osmosis High To Low Or Low To High
Mar 15, 2025
-
Concave Mirror And Convex Mirror Difference
Mar 15, 2025
-
Which Is Not A Cranial Bone Of The Skull
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Iupac Name Of The Compound Shown Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
