What Is The Ideal Van't Hoff Factor For Glucose
News Leon
Mar 17, 2025 · 5 min read
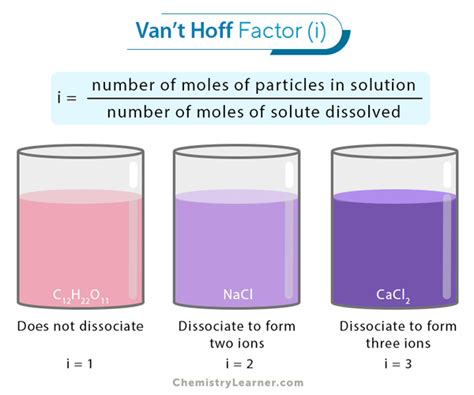
Table of Contents
What is the Ideal Van't Hoff Factor for Glucose?
The van't Hoff factor (i) is a crucial concept in chemistry, particularly when dealing with colligative properties of solutions. It represents the ratio of the actual concentration of particles produced when a substance dissolves to the concentration of the substance as calculated from its mass. Understanding the van't Hoff factor is essential for accurately predicting phenomena like osmotic pressure, freezing point depression, and boiling point elevation. This article delves into the ideal van't Hoff factor for glucose, exploring its theoretical value, deviations from ideality, and the factors influencing these deviations.
Understanding the Van't Hoff Factor
The van't Hoff factor is named after Jacobus Henricus van't Hoff, a Nobel laureate who made significant contributions to physical chemistry. It's a dimensionless quantity that reflects the extent to which a solute dissociates or associates in a solution.
-
For non-electrolytes: Non-electrolytes, like glucose, do not dissociate into ions when dissolved in a solvent. Their ideal van't Hoff factor is 1. This means that one molecule of the solute produces one particle in the solution.
-
For electrolytes: Electrolytes, such as salts, dissociate into ions in solution. Their van't Hoff factor is greater than 1, and its value depends on the number of ions produced per formula unit. For example, NaCl (sodium chloride) has an ideal van't Hoff factor of 2 because it dissociates into two ions (Na⁺ and Cl⁻).
The Ideal Van't Hoff Factor for Glucose: A Deep Dive
Glucose (C₆H₁₂O₆) is a non-electrolyte. It dissolves in water to form a molecular solution, meaning it does not dissociate into ions. Therefore, the ideal van't Hoff factor for glucose is 1. This is because each glucose molecule remains as a single entity in the aqueous solution, contributing only one particle to the total number of dissolved particles.
Theoretical Calculation of the Van't Hoff Factor for Glucose
The theoretical calculation for glucose is straightforward. Since it doesn't dissociate, the number of particles in solution is equal to the number of glucose molecules dissolved. Hence, the ratio (actual concentration of particles / concentration of glucose) simplifies to 1.
Experimental Determination of the Van't Hoff Factor
While the theoretical value for glucose is 1, experimental determination might show slight deviations. This is because the actual behavior of molecules in solution is often more complex than idealized models predict. These deviations are usually small for glucose in dilute solutions.
Deviations from Ideality: Why the Experimental Value Might Differ
Although the ideal van't Hoff factor for glucose is 1, experimental values might slightly deviate from this ideal. These deviations arise from several factors:
-
Intermolecular interactions: Glucose molecules can interact with each other through hydrogen bonding and van der Waals forces. These interactions can affect their effective concentration and consequently the measured colligative properties. Stronger interactions can lead to a slightly lower effective concentration of glucose particles, resulting in a van't Hoff factor slightly less than 1. However, these deviations are typically minor in dilute solutions.
-
Hydration: Glucose molecules can interact with water molecules, forming hydration shells. These hydrated glucose molecules behave differently than free glucose molecules, influencing the colligative properties and potentially causing a slight deviation from the ideal value.
-
Concentration effects: In concentrated solutions, the interactions between solute molecules (glucose) become more significant. These interactions can lead to a deviation from ideality, and the van't Hoff factor may deviate from 1. In dilute solutions, however, these effects are minimized.
-
Temperature and pressure: Temperature and pressure can also affect the interactions between glucose molecules and water molecules, indirectly influencing the van't Hoff factor. Higher temperatures can increase the kinetic energy of the molecules, disrupting interactions and potentially leading to values closer to 1.
-
Experimental errors: Experimental measurements always involve some degree of error. Inaccurate measurements of concentration, temperature, or colligative properties can lead to deviations from the ideal van't Hoff factor.
Practical Applications of the Van't Hoff Factor for Glucose
Understanding the van't Hoff factor for glucose has practical applications in various fields:
-
Pharmaceutical industry: Determining the osmotic pressure of glucose solutions is crucial in pharmaceutical formulations to ensure compatibility with biological systems. The van't Hoff factor is essential for accurate calculations.
-
Food science: Glucose solutions are used extensively in the food industry. Understanding colligative properties, influenced by the van't Hoff factor, is crucial for controlling the texture and properties of food products.
-
Biochemistry and cell biology: Glucose plays a vital role in biological systems. Understanding its behavior in solution, including its van't Hoff factor, is essential for studying cellular processes and transport mechanisms.
-
Analytical chemistry: The van't Hoff factor is used in various analytical techniques to determine the molar mass and concentration of glucose in solutions.
Distinguishing Between Ideal and Actual Van't Hoff Factors
It is crucial to emphasize the difference between the ideal and the actual van't Hoff factor. The ideal value reflects the theoretical behavior of the solute based on its dissociation or association. The actual value, determined experimentally, accounts for deviations from ideality caused by various factors like intermolecular interactions and concentration effects. For glucose, while the ideal is 1, the actual might show minor deviations, especially in concentrated solutions.
Conclusion: The Importance of Understanding Deviations
The ideal van't Hoff factor for glucose is 1, reflecting its non-electrolytic nature and lack of dissociation in solution. However, the actual van't Hoff factor might differ slightly from this ideal value due to various factors like intermolecular interactions, hydration, concentration effects, and experimental errors. Understanding these deviations is crucial for accurate calculations and predictions related to colligative properties in glucose solutions, impacting various scientific and industrial applications. In dilute solutions, however, the deviation from ideality is minimal, and using the ideal value of 1 is a reasonable approximation for most practical purposes. Always consider the experimental conditions and the specific application when interpreting the van't Hoff factor for glucose. Further research and advanced experimental techniques continue to refine our understanding of the intricacies of solute behavior in solution.
Latest Posts
Latest Posts
-
8 Is 20 Of What Number
Mar 18, 2025
-
Predict What Is Present In Each Of The Following
Mar 18, 2025
-
Which Of The Following Word Is Different From The Others
Mar 18, 2025
-
Reverse List Python Without Inbuilt Function
Mar 18, 2025
-
A Current Of One Ampere Is Passed Through
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Ideal Van't Hoff Factor For Glucose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
