What Is The Freezing Point For Water In Celsius
News Leon
Mar 26, 2025 · 6 min read
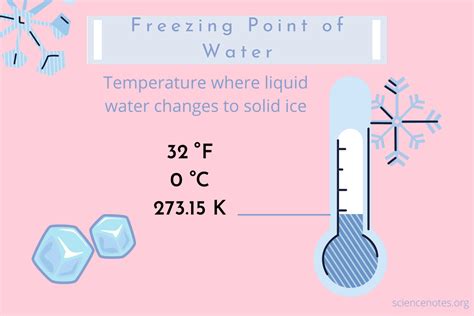
Table of Contents
What is the Freezing Point of Water in Celsius? A Deep Dive into Water's Properties
Water, the elixir of life, is a substance so ubiquitous that we often take its remarkable properties for granted. One of the most fundamental aspects of water, crucial to countless processes on Earth and vital to our understanding of chemistry and physics, is its freezing point. So, what is the freezing point of water in Celsius? The simple answer is 0°C. But this seemingly straightforward answer opens the door to a fascinating exploration of the science behind this critical temperature point.
Understanding the Freezing Point: A Molecular Perspective
The freezing point of water, or its melting point (the temperature at which it transitions from solid to liquid), represents the temperature at which the kinetic energy of water molecules is low enough to allow the formation of a stable crystalline structure—ice. At temperatures above 0°C, water molecules possess sufficient kinetic energy to overcome the attractive forces holding them together in a rigid structure. They move freely, exhibiting the fluidity characteristic of liquid water.
However, as the temperature drops towards 0°C, the kinetic energy of the molecules decreases. This allows the intermolecular forces, primarily hydrogen bonds, to dominate. Hydrogen bonds are relatively strong electrostatic attractions between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another. These bonds are responsible for many of water's unique properties, including its high boiling point, surface tension, and, crucially, its relatively high freezing point compared to similar molecules.
As the temperature reaches 0°C, the water molecules slow down sufficiently to form a stable, ordered lattice structure—the characteristic hexagonal crystalline structure of ice. This transition from a disordered liquid to an ordered solid releases energy, known as the latent heat of fusion. This energy must be removed from the water for freezing to occur. Conversely, the same amount of energy is required to melt ice at 0°C.
Factors Affecting the Freezing Point of Water
While 0°C is the standard freezing point of pure water at standard atmospheric pressure (1 atm), several factors can influence this temperature:
-
Pressure: Increasing the pressure on water actually lowers its freezing point. This seemingly counterintuitive phenomenon is due to the fact that ice is less dense than liquid water (a unique property). Applying pressure favors the denser liquid phase, thus requiring a slightly lower temperature for freezing to occur. This effect is relatively small at pressures near atmospheric pressure but becomes more significant at higher pressures.
-
Impurities: Dissolved substances, such as salts or sugars, lower the freezing point of water. This phenomenon is known as freezing point depression. The extent of the depression depends on the concentration of the dissolved substance. This is why salt is used to de-ice roads and sidewalks in winter. The salt dissolves in the thin layer of water on the surface, lowering its freezing point and preventing ice formation.
-
Isotopes: The presence of different isotopes of hydrogen and oxygen in water molecules can also subtly affect the freezing point. Heavy water (D₂O), containing deuterium instead of ordinary hydrogen, has a freezing point of approximately 3.8°C.
The Importance of 0°C in Various Applications
The precise knowledge of water's freezing point at 0°C is fundamental across numerous scientific disciplines and practical applications:
-
Meteorology: Understanding the freezing point is crucial for weather forecasting and climate modeling. Freezing temperatures trigger the formation of ice crystals in clouds, leading to precipitation like snow and hail. Accurately predicting freezing temperatures is vital for protecting crops, infrastructure, and public safety.
-
Biology: The freezing point of water is directly related to the survival strategies of organisms in cold environments. Many organisms have evolved mechanisms to prevent ice formation within their cells, protecting themselves from damage caused by ice crystal formation. Conversely, the freezing and thawing of water plays a critical role in processes like soil formation and nutrient cycling in ecosystems.
-
Chemistry: The freezing point of water serves as a fundamental reference point in various chemical analyses and experiments. It is used in calibrating thermometers and other temperature-measuring devices, ensuring accurate measurements in countless chemical processes.
-
Engineering: Engineers need to consider the freezing point of water when designing and constructing structures in cold climates. Proper insulation and materials selection are essential to prevent damage caused by freezing and thawing cycles. This is particularly crucial for infrastructure like pipelines, roads, and buildings.
-
Food Science: The freezing point of water is critical in food preservation. Freezing food slows down or stops microbial growth, extending shelf life. Understanding the freezing point of water and its impact on food texture and quality is essential for developing effective freezing and thawing methods.
-
Material Science: Many materials are affected by the presence of water, and understanding its freezing point is critical in designing and manufacturing materials that are resistant to damage from freezing and thawing. This is relevant in areas like construction, aerospace, and electronics.
Beyond the Basics: Anomalies and Further Explorations
While 0°C is the widely accepted freezing point of water, understanding the nuanced aspects of water's behavior near its freezing point reveals interesting anomalies and areas for ongoing scientific investigation:
-
Supercooling: Under certain conditions, water can remain in its liquid state even below 0°C, a phenomenon known as supercooling. This typically occurs in the absence of nucleation sites—surfaces or impurities that facilitate the formation of ice crystals. Supercooled water is metastable, meaning it's in a state of temporary equilibrium, and a slight disturbance can trigger rapid freezing.
-
Ice Polymorphism: Water can form different crystalline structures (polymorphs) of ice, each with slightly different properties. Ordinary ice (Ice Ih) is the most common form, but at least 17 other ice polymorphs have been identified, some of which are stable under high pressure or low temperatures. The study of these different ice forms is essential for understanding water's behavior in extreme conditions.
-
Water's Anomalous Density: The fact that ice is less dense than liquid water is unusual. Most substances become denser upon freezing. This anomalous density of ice is crucial for life on Earth, as it allows ice to float on water, insulating aquatic life from freezing temperatures. The research into this anomaly and its implications remains an active field.
Conclusion: The Significance of a Simple Number
The freezing point of water at 0°C is more than just a simple number; it's a fundamental constant with profound implications across diverse scientific disciplines and practical applications. Understanding this temperature and the factors that influence it is critical for addressing numerous challenges related to weather forecasting, biology, engineering, and numerous other fields. Further research into the nuances of water's behavior near its freezing point continues to unlock new insights into this remarkable substance and its critical role in our world. The seemingly simple question—what is the freezing point of water in Celsius?—leads us to a far deeper and more complex exploration of the wonders of water itself.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Lymphoid Organ
Mar 26, 2025
-
What Is The Reason For Doing A Test Cross
Mar 26, 2025
-
Is The Following Relation A Function
Mar 26, 2025
-
Which Of The Following Is The Smallest Unit Of Measurement
Mar 26, 2025
-
When Lithium Hydroxide Pellets Are Added
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Freezing Point For Water In Celsius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
