What Is The Freezing Point For Kelvin
News Leon
Mar 30, 2025 · 6 min read
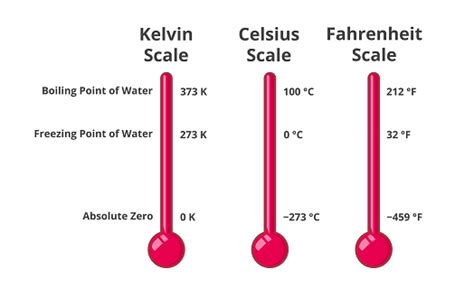
Table of Contents
What is the Freezing Point of Kelvin? Understanding Absolute Zero and the Kelvin Scale
The question "What is the freezing point of Kelvin?" might seem straightforward, but it highlights a crucial misunderstanding about the Kelvin scale. Unlike Celsius and Fahrenheit, which are relative scales based on the freezing and boiling points of water, Kelvin is an absolute temperature scale. This means it doesn't have a "freezing point" in the same way. Instead, it starts at absolute zero, the theoretical point where all molecular motion ceases. This article will delve into a comprehensive explanation of the Kelvin scale, its relationship to other temperature scales, and why the concept of a "freezing point" doesn't apply.
Understanding the Kelvin Scale: A Foundation in Absolute Zero
The Kelvin scale, named after Lord Kelvin (William Thomson), is a fundamental scale in thermodynamics and physics. Its defining characteristic is its absolute zero point, representing the lowest possible temperature. At this point, 0 Kelvin (0 K), all matter has minimal kinetic energy; its particles are essentially motionless. This isn't merely the absence of heat; it's the theoretical limit of coldness.
Absolute Zero: The Theoretical Limit
While scientists can get incredibly close to absolute zero through advanced techniques like laser cooling, they can never actually reach it. The laws of thermodynamics prevent achieving true absolute zero. The closer you get, the more energy is required to approach it further, making it an asymptotic limit.
The Relationship Between Kelvin and Celsius
The Kelvin scale is directly related to the Celsius scale. The size of a Kelvin degree is the same as the size of a Celsius degree. The only difference is the zero point:
- 0 Kelvin (0 K) = -273.15 degrees Celsius (°C)
This means you can easily convert between Kelvin and Celsius using the following formulas:
- K = °C + 273.15 (Kelvin to Celsius)
- °C = K - 273.15 (Celsius to Kelvin)
Why the Freezing Point Concept Doesn't Apply to Kelvin
The concept of a "freezing point" implies a transition from a liquid to a solid state. This transition is specific to the substance in question and depends on factors like pressure. While water freezes at 0 °C (273.15 K) at standard atmospheric pressure, other substances freeze at different temperatures.
The Kelvin scale focuses on the total energy of a system. It doesn't define a specific phase transition like freezing. Therefore, asking for the "freezing point" of Kelvin is akin to asking for the "boiling point" of meters – it's a fundamentally incompatible question. Kelvin measures the absolute energy, whereas freezing points refer to specific phase transitions related to the molecular structure and intermolecular forces of the substance.
Practical Applications of the Kelvin Scale
Despite not having a "freezing point," the Kelvin scale is critical in numerous scientific and engineering applications. Its absolute nature makes it ideal for:
1. Thermodynamics and Statistical Mechanics
The Kelvin scale is fundamental in thermodynamics, where it's used to define concepts like entropy, enthalpy, and Gibbs free energy. These concepts rely on absolute temperature values rather than relative ones.
2. Gas Laws and Ideal Gas Behavior
Gas laws, such as the ideal gas law (PV=nRT), use Kelvin temperature to accurately predict the behavior of gases. Using Celsius or Fahrenheit would lead to inaccurate results, especially at low temperatures.
3. Astrophysics and Cosmology
In astrophysics, the Kelvin scale is used to measure the temperature of stars, planets, and other celestial bodies. The incredibly high temperatures involved require an absolute scale for accurate measurements and comparisons.
4. Cryogenics and Low-Temperature Physics
Cryogenics, the study of extremely low temperatures, relies heavily on the Kelvin scale. Researchers working near absolute zero use Kelvin to track subtle temperature changes with extreme precision.
5. Material Science and Engineering
Understanding the behavior of materials at various temperatures is crucial in material science and engineering. The Kelvin scale helps to define phase transitions and other temperature-dependent properties of materials accurately.
Comparing Kelvin to Other Temperature Scales
Let's briefly compare the Kelvin scale to the Celsius and Fahrenheit scales to further illustrate its unique nature:
Celsius (°C)
- Zero point: Defined as the freezing point of water at standard atmospheric pressure.
- Relative scale: Based on the freezing and boiling points of water.
- Used for: Everyday temperature measurements, scientific applications (especially in chemistry and biology).
Fahrenheit (°F)
- Zero point: Arbitrarily defined based on a brine freezing point.
- Relative scale: Like Celsius, it's based on a range defined by water's properties.
- Used for: Primarily in the United States for weather reporting and everyday purposes.
Kelvin (K)
- Zero point: Absolute zero, the point where all molecular motion ceases.
- Absolute scale: Not based on any substance's properties; it's based on energy.
- Used for: Scientific applications where absolute temperature is crucial, such as thermodynamics, astrophysics, and cryogenics.
The table below summarizes the key differences:
| Feature | Kelvin (K) | Celsius (°C) | Fahrenheit (°F) |
|---|---|---|---|
| Zero Point | Absolute zero | Freezing point of water | Arbitrary point |
| Scale Type | Absolute | Relative | Relative |
| Water Freezing | 273.15 K | 0 °C | 32 °F |
| Water Boiling | 373.15 K | 100 °C | 212 °F |
| Unit Size | Same as °C | N/A | Smaller than °C |
Beyond the Freezing Point: Exploring Other Temperature-Related Concepts
While the concept of a freezing point doesn't apply directly to the Kelvin scale, understanding other temperature-related concepts enhances our grasp of its significance:
1. Phase Transitions:
Phase transitions, like melting, boiling, and sublimation, are temperature-dependent. Although Kelvin doesn't have a specific freezing point, it's used to accurately determine the temperature at which these transitions occur for various substances.
2. Thermal Expansion:
Most materials expand when heated and contract when cooled. The Kelvin scale is crucial in calculating the extent of this thermal expansion and is often utilized in engineering designs to account for thermal stress.
3. Heat Capacity and Specific Heat:
Heat capacity and specific heat represent the amount of heat required to change the temperature of a substance. Calculations involving these properties use Kelvin to ensure accuracy.
4. Blackbody Radiation:
Blackbody radiation, the electromagnetic radiation emitted by an idealized object that absorbs all incident radiation, is described by Planck's law, which uses temperature in Kelvin.
Conclusion: The Significance of the Kelvin Scale
In conclusion, the Kelvin scale doesn't possess a "freezing point" in the traditional sense. Its significance lies in its absolute nature, making it invaluable in scientific disciplines where precise temperature measurements and understanding the fundamental relationships between temperature and other physical phenomena are paramount. The absolute zero point of the Kelvin scale represents a theoretical limit, providing a foundation for various thermodynamic concepts and calculations across diverse scientific and engineering fields. Understanding the differences between the Kelvin, Celsius, and Fahrenheit scales is essential for anyone working with temperature data in any scientific or engineering endeavor. The Kelvin scale's enduring importance in scientific research and applications confirms its indispensable role in our understanding of the physical world.
Latest Posts
Latest Posts
-
How Many Protons Are There In Any Chlorine Atom
Apr 01, 2025
-
Which Of The Following Is Not A Monomial
Apr 01, 2025
-
Which Of The Following Is Not A Lymphatic Organ
Apr 01, 2025
-
An Astronauts Weight On Earth Is 800 N
Apr 01, 2025
-
What Is The Percent Of 13 20
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Freezing Point For Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
