What Is The Empirical Formula Of Ibuprofen
News Leon
Mar 26, 2025 · 5 min read
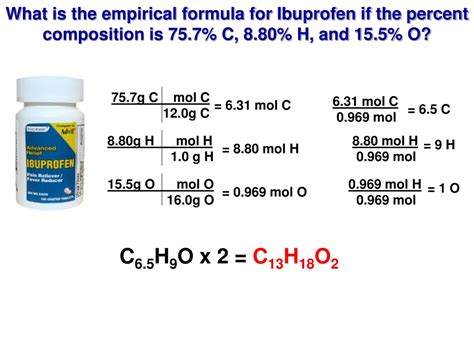
Table of Contents
What is the Empirical Formula of Ibuprofen? A Deep Dive into Molecular Structure and Determination
Ibuprofen, a widely used nonsteroidal anti-inflammatory drug (NSAID), is a familiar household name for its effectiveness in relieving pain, fever, and inflammation. But beyond its medicinal properties, understanding its chemical composition, particularly its empirical formula, offers a fascinating glimpse into the world of organic chemistry. This article will delve into the determination of ibuprofen's empirical formula, exploring the concepts of molecular formulas, empirical formulas, and the process of deriving one from the other. We'll also touch upon related concepts like molar mass and percentage composition, crucial for understanding the substance's chemical identity.
Understanding Empirical and Molecular Formulas
Before we tackle ibuprofen specifically, let's clarify the difference between empirical and molecular formulas.
-
Molecular Formula: This formula represents the actual number of atoms of each element present in a single molecule of a compound. For instance, the molecular formula of glucose is C₆H₁₂O₆, indicating six carbon atoms, twelve hydrogen atoms, and six oxygen atoms in one molecule.
-
Empirical Formula: This formula represents the simplest whole-number ratio of atoms of each element in a compound. It's the most reduced form of the molecular formula. For glucose (C₆H₁₂O₆), the empirical formula is CH₂O, representing the 1:2:1 ratio of carbon, hydrogen, and oxygen atoms. Note that many compounds may share the same empirical formula but have different molecular formulas.
Determining the Empirical Formula: A Step-by-Step Approach
The determination of an empirical formula generally relies on experimental data, usually obtained through techniques like combustion analysis. This involves completely burning a known mass of the compound and measuring the masses of the resulting products (e.g., carbon dioxide and water). However, for the purposes of this article, we'll assume we have access to the molecular formula of ibuprofen and then derive its empirical formula.
Ibuprofen's Molecular Formula and Structure
The molecular formula of ibuprofen is C₁₃H₁₈O₂. This tells us that one molecule of ibuprofen contains 13 carbon atoms, 18 hydrogen atoms, and 2 oxygen atoms. The structural formula adds a spatial dimension, illustrating how these atoms are bonded together:
(Image of Ibuprofen's structural formula would be inserted here. Since I cannot create images, please imagine a detailed structural formula showing the arrangement of atoms and bonds in ibuprofen.)
Deriving Ibuprofen's Empirical Formula
To find the empirical formula from the molecular formula (C₁₃H₁₈O₂), we need to determine the greatest common divisor (GCD) of the subscripts. In this case, the subscripts are 13, 18, and 2. The GCD of these numbers is 1. Since the GCD is 1, the empirical formula is the same as the molecular formula: C₁₃H₁₈O₂. This means that the simplest whole-number ratio of carbon, hydrogen, and oxygen atoms in ibuprofen is 13:18:2.
Molar Mass and Percentage Composition: Further Insights
While we've determined the empirical formula, understanding ibuprofen's molar mass and percentage composition provides a more complete picture of its chemical makeup.
- Molar Mass: The molar mass of ibuprofen (C₁₃H₁₈O₂) is calculated by summing the atomic masses of its constituent atoms. Using approximate atomic masses (C = 12.01 g/mol, H = 1.01 g/mol, O = 16.00 g/mol), we get:
(13 * 12.01 g/mol) + (18 * 1.01 g/mol) + (2 * 16.00 g/mol) ≈ 206.29 g/mol
-
Percentage Composition: The percentage composition indicates the mass percentage of each element in the compound. We can calculate this using the molar mass of each element and the total molar mass of ibuprofen:
-
% Carbon: [(13 * 12.01 g/mol) / 206.29 g/mol] * 100% ≈ 75.7%
-
% Hydrogen: [(18 * 1.01 g/mol) / 206.29 g/mol] * 100% ≈ 8.8%
-
% Oxygen: [(2 * 16.00 g/mol) / 206.29 g/mol] * 100% ≈ 15.5%
These values show that carbon makes up the largest portion of ibuprofen's mass, followed by hydrogen and then oxygen.
Significance of Empirical Formula in Pharmaceutical Chemistry
Knowing the empirical formula of ibuprofen, and other pharmaceutical compounds, is crucial for several reasons:
-
Quality Control: Pharmaceutical manufacturers use empirical formulas to verify the purity and consistency of their products. Deviation from the expected empirical formula could indicate impurities or manufacturing errors.
-
Dosage Calculations: The empirical formula, combined with molar mass, is essential for accurate dosage calculations and formulation of pharmaceutical preparations.
-
Drug Development: Understanding the composition of a drug allows scientists to modify and optimize its structure for improved efficacy and reduced side effects.
Beyond Ibuprofen: Applications in Other Fields
The concept of empirical formulas extends far beyond pharmaceuticals. It's fundamental in various fields, including:
-
Materials Science: Determining the empirical formula of newly synthesized materials helps in understanding their properties and potential applications.
-
Environmental Science: Analyzing the empirical formulas of pollutants allows for better understanding of their environmental impact and development of remediation strategies.
-
Forensic Science: Determining the empirical formulas of unknown substances found at crime scenes can provide crucial clues in investigations.
Conclusion
The empirical formula of ibuprofen, C₁₃H₁₈O₂, is a fundamental piece of information that sheds light on its chemical composition and properties. While the empirical formula in this case happens to be identical to the molecular formula, understanding the difference between these two types of formulas is critical in chemistry. The methods used to determine empirical formulas are widely applied in various scientific disciplines, highlighting the importance of this basic concept in our quest to understand the composition of matter. The determination of empirical formula, along with molar mass and percentage composition, is not merely an academic exercise but a fundamental tool in various fields, directly impacting our daily lives through drug development, materials science, and environmental monitoring.
Latest Posts
Latest Posts
-
In Which Stage Of Meiosis Crossing Over Occurs
Mar 26, 2025
-
Which Of The Following Is Not A Lymphoid Organ
Mar 26, 2025
-
What Is The Reason For Doing A Test Cross
Mar 26, 2025
-
Is The Following Relation A Function
Mar 26, 2025
-
Which Of The Following Is The Smallest Unit Of Measurement
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Empirical Formula Of Ibuprofen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
