What Is The Empirical Formula Of Glucose
News Leon
Mar 16, 2025 · 5 min read
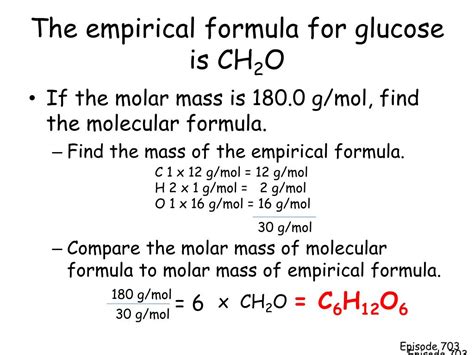
Table of Contents
What is the Empirical Formula of Glucose? A Deep Dive into Molecular Composition
Glucose, a simple sugar and the primary source of energy for living organisms, holds a significant place in biochemistry and organic chemistry. Understanding its chemical composition, particularly its empirical formula, is crucial for grasping its role in various biological processes. This article delves into the intricacies of glucose's molecular structure, explaining what an empirical formula is, how it differs from the molecular formula, and how we determine the empirical formula of glucose through experimental techniques and calculations. We'll also explore the significance of understanding glucose's empirical formula in various scientific fields.
Understanding Empirical Formulas
Before we dive into the specifics of glucose, let's clarify the concept of an empirical formula. An empirical formula represents the simplest whole-number ratio of atoms of each element present in a compound. It doesn't necessarily indicate the actual number of atoms in a molecule, but rather the ratio between them. This is different from the molecular formula, which provides the exact number of each type of atom in a molecule.
For example, consider hydrogen peroxide (H₂O₂). Its molecular formula clearly shows two hydrogen atoms and two oxygen atoms. However, its empirical formula is simply HO, reflecting the 1:1 ratio of hydrogen to oxygen atoms. The empirical formula provides the most basic representation of the compound's elemental composition.
Determining the Empirical Formula: A Step-by-Step Guide
The determination of an empirical formula typically involves experimental techniques to determine the mass percentage of each element in the compound. This data is then used to calculate the mole ratio of the elements, leading to the simplest whole-number ratio represented by the empirical formula. Here's a general approach:
-
Elemental Analysis: The first step involves accurately determining the percentage composition by mass of each element in the glucose sample. This can be achieved using various analytical techniques, such as combustion analysis. Combustion analysis involves completely burning a known mass of the glucose sample in excess oxygen. The resulting products – carbon dioxide (CO₂) and water (H₂O) – are collected and weighed. From the masses of CO₂ and H₂O, the masses of carbon and hydrogen in the original glucose sample can be calculated. The mass of oxygen can then be determined by difference (total mass of glucose – mass of carbon – mass of hydrogen).
-
Converting Mass to Moles: Once the mass of each element is known, it's converted to moles using the element's molar mass (atomic weight). The molar mass of carbon is approximately 12.01 g/mol, hydrogen is approximately 1.01 g/mol, and oxygen is approximately 16.00 g/mol. The number of moles of each element is calculated using the formula:
Moles = Mass (g) / Molar Mass (g/mol)
-
Determining the Mole Ratio: The next crucial step is to determine the mole ratio of the elements. This involves dividing the number of moles of each element by the smallest number of moles calculated in the previous step. This will provide a ratio of the elements in the simplest whole-number form.
-
Expressing the Empirical Formula: Finally, the empirical formula is expressed using the whole-number ratios obtained in the previous step. The subscripts in the empirical formula represent these ratios.
Empirical Formula of Glucose: The Calculation
Let's assume, for the sake of illustration, that combustion analysis of a glucose sample yielded the following mass percentages:
- Carbon (C): 40%
- Hydrogen (H): 6.7%
- Oxygen (O): 53.3%
To calculate the empirical formula, we follow the steps outlined above:
-
Assume 100g of Glucose: For easier calculation, we assume a 100g sample of glucose. This means we have 40g of carbon, 6.7g of hydrogen, and 53.3g of oxygen.
-
Convert to Moles:
- Moles of Carbon: 40g / 12.01 g/mol ≈ 3.33 mol
- Moles of Hydrogen: 6.7g / 1.01 g/mol ≈ 6.63 mol
- Moles of Oxygen: 53.3g / 16.00 g/mol ≈ 3.33 mol
-
Determine Mole Ratio: Divide each number of moles by the smallest number of moles (3.33 mol):
- Carbon: 3.33 mol / 3.33 mol = 1
- Hydrogen: 6.63 mol / 3.33 mol ≈ 2
- Oxygen: 3.33 mol / 3.33 mol = 1
-
Empirical Formula: The empirical formula of glucose is therefore CH₂O.
Empirical Formula vs. Molecular Formula of Glucose
While the empirical formula of glucose is CH₂O, its molecular formula is C₆H₁₂O₆. This means that a glucose molecule contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. The molecular formula is a multiple of the empirical formula (CH₂O)₆. Determining the molecular formula requires additional information, such as the molar mass of glucose, which can be determined experimentally or obtained from a database.
Significance of Understanding Glucose's Empirical Formula
Understanding the empirical formula of glucose is significant in various scientific fields:
-
Biochemistry: The empirical formula provides a simplified representation of glucose's elemental composition, crucial for understanding its metabolic pathways and its role in energy production.
-
Organic Chemistry: The empirical formula helps in the identification and characterization of glucose, distinguishing it from other carbohydrates with similar empirical formulas but different molecular structures.
-
Analytical Chemistry: The determination of glucose's empirical formula relies on precise analytical techniques, emphasizing the importance of accurate measurements and data analysis in chemical investigations.
-
Food Science and Nutrition: Understanding the elemental composition of glucose is essential in food science and nutrition, contributing to the analysis of carbohydrate content in various foods.
-
Medical Science: In medical science, understanding glucose metabolism is vital for diagnosing and managing diabetes and other metabolic disorders. The empirical formula provides a basic understanding of the composition of this vital biomolecule.
Conclusion
The empirical formula of glucose, CH₂O, while a simplified representation, is a cornerstone for understanding its chemical nature and biological functions. By employing experimental techniques like combustion analysis and subsequent calculations, we can determine this fundamental ratio of elements within the molecule. While it doesn't provide the complete picture, the empirical formula serves as a crucial stepping stone toward a more comprehensive understanding of glucose's molecular structure and its profound role in biological systems. Knowing the empirical formula provides a basis for further investigation into its properties and reactions, contributing to advancements in various scientific disciplines. Remember, the empirical formula offers a simplified, yet powerful, insight into the composition of this vital biomolecule.
Latest Posts
Latest Posts
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Empirical Formula Of Glucose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
