What Is The Electron Configuration Of Titanium
News Leon
Mar 25, 2025 · 6 min read
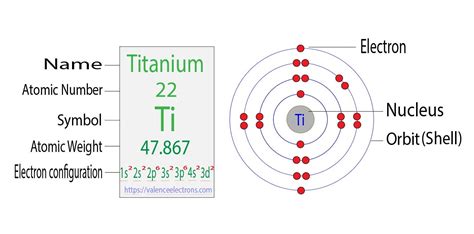
Table of Contents
- What Is The Electron Configuration Of Titanium
- Table of Contents
- What is the Electron Configuration of Titanium? A Deep Dive into Atomic Structure
- Understanding Electron Configuration
- The Electron Configuration of Titanium (Ti)
- The Significance of the 4s and 3d Orbitals
- Titanium Ions and Electron Configuration
- The Impact of Electron Configuration on Titanium's Properties
- Applications Driven by Titanium's Electron Configuration
- Further Exploration: Excited States and Spectroscopic Studies
- Conclusion: The Foundation of Titanium's Uniqueness
- Latest Posts
- Latest Posts
- Related Post
What is the Electron Configuration of Titanium? A Deep Dive into Atomic Structure
Titanium, a lustrous transition metal with the symbol Ti and atomic number 22, boasts a fascinating electron configuration that underpins its unique properties and reactivity. Understanding its electron configuration is key to comprehending its behavior in various chemical and physical contexts. This article provides a comprehensive exploration of titanium's electron configuration, delving into its implications for the element's characteristics and applications.
Understanding Electron Configuration
Before diving into titanium's specifics, let's establish a foundational understanding of electron configuration. The electron configuration of an atom describes how electrons are distributed among the various energy levels (shells) and sublevels (subshells) within the atom. This arrangement dictates the atom's chemical properties, its ability to form bonds, and its overall reactivity. Electrons occupy these shells and subshells according to the Aufbau principle, filling lower energy levels first before moving to higher ones. The Pauli exclusion principle states that each orbital can hold a maximum of two electrons, each with opposite spins. Finally, Hund's rule dictates that electrons will individually occupy each orbital within a subshell before pairing up.
We represent electron configurations using a shorthand notation that indicates the principal quantum number (n), the type of subshell (s, p, d, or f), and the number of electrons in each subshell. For example, 1s² means two electrons in the first energy level's s subshell.
The Electron Configuration of Titanium (Ti)
Titanium has an atomic number of 22, meaning it possesses 22 protons and, in its neutral state, 22 electrons. Following the Aufbau principle, Hund's rule, and the Pauli exclusion principle, the ground state electron configuration of titanium is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d²
Let's break this down:
- 1s²: Two electrons in the first energy level's s subshell.
- 2s²: Two electrons in the second energy level's s subshell.
- 2p⁶: Six electrons in the second energy level's p subshell (p subshells can hold up to six electrons).
- 3s²: Two electrons in the third energy level's s subshell.
- 3p⁶: Six electrons in the third energy level's p subshell.
- 4s²: Two electrons in the fourth energy level's s subshell.
- 3d²: Two electrons in the third energy level's d subshell.
This configuration highlights titanium's position as a transition metal. Transition metals are characterized by partially filled d subshells. The presence of two electrons in the 3d subshell is a defining feature of titanium's electron configuration and explains many of its properties.
The Significance of the 4s and 3d Orbitals
The order of filling orbitals isn't always strictly sequential. While the Aufbau principle generally guides the filling order, exceptions exist, particularly among transition metals. In the case of titanium, the 4s subshell fills before the 3d subshell. This is due to subtle differences in the energy levels of these subshells, making the 4s slightly lower in energy than the 3d in neutral titanium. This slight energy difference explains why the 4s orbital is filled first.
However, it's crucial to understand that the energy difference between the 4s and 3d orbitals is relatively small. This small energy difference allows for flexibility in electron arrangements, especially when titanium forms ions.
Titanium Ions and Electron Configuration
When titanium forms ions, it loses electrons from its outermost shells. Titanium commonly forms Ti²⁺ and Ti⁴⁺ ions. These ionic forms have different electron configurations:
- Ti²⁺: Loses the two 4s electrons, resulting in the configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d²
- Ti⁴⁺: Loses two 4s electrons and two 3d electrons, resulting in the configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ This configuration is isoelectronic with Argon, a noble gas. This fully filled electron shell is exceptionally stable, explaining the relatively high stability of the Ti⁴⁺ ion.
The ability of titanium to form stable ions with different charges contributes to its versatility in chemical reactions and its capacity to form a wide range of compounds.
The Impact of Electron Configuration on Titanium's Properties
Titanium's unique electron configuration is directly responsible for many of its physical and chemical properties:
- High Melting Point: The strong metallic bonding arising from the electron configuration contributes to a relatively high melting point of approximately 1668 °C.
- Strength and Light Weight: Titanium exhibits an excellent strength-to-weight ratio, making it a highly desirable material in aerospace and other high-performance applications. This property is a direct consequence of its metallic bonding and crystal structure.
- Corrosion Resistance: Titanium's ability to form a stable and protective oxide layer on its surface renders it highly resistant to corrosion, even in aggressive environments. This passivation layer is related to its electron configuration and its ability to readily share electrons.
- Biocompatibility: Titanium's inertness and biocompatibility make it ideal for medical implants and surgical instruments. Its electron configuration contributes to its lack of toxicity and its inertness in biological environments.
- Reactivity: Although generally unreactive at room temperature due to the formation of its protective oxide layer, titanium can react with many elements under certain conditions. This reactivity is linked to its electron configuration and the ability of the d electrons to participate in chemical bonding.
Applications Driven by Titanium's Electron Configuration
The unique characteristics derived from its electron configuration make titanium crucial in various industries:
- Aerospace: Titanium alloys are extensively used in aircraft and spacecraft construction due to their high strength-to-weight ratio and corrosion resistance.
- Medical Implants: The biocompatibility of titanium makes it ideal for surgical implants, such as hip replacements and dental implants.
- Chemical Processing: Titanium's resistance to corrosion allows for its use in handling corrosive chemicals.
- Sporting Goods: Titanium's strength and lightweight properties find applications in bicycles, golf clubs, and other sporting goods.
- Jewelry: Its lustrous appearance and resistance to tarnish make it a popular choice for jewelry.
Further Exploration: Excited States and Spectroscopic Studies
While the ground state electron configuration is the most stable arrangement, titanium, like any atom, can exist in excited states where electrons occupy higher energy levels. These excited states can be studied using spectroscopic techniques, providing detailed information about the energy levels and transitions within the atom. This information is valuable in understanding the atom’s behavior and its interactions with light.
Conclusion: The Foundation of Titanium's Uniqueness
The electron configuration of titanium, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d², is the fundamental blueprint that dictates its remarkable properties and wide-ranging applications. Understanding this configuration allows us to appreciate the intricate relationship between atomic structure and macroscopic behavior. The partially filled d subshell, the relatively small energy difference between the 4s and 3d orbitals, and the formation of stable ions are all key factors contributing to titanium's unique position in the periodic table and its immense technological importance. This detailed exploration has unveiled not just the configuration itself, but also the profound implications it holds for understanding this essential element. From its use in aerospace marvels to its role in life-saving medical implants, titanium’s story is intricately woven with the fundamental principles of atomic structure and the elegance of its electron configuration.
Latest Posts
Latest Posts
-
Linear Mass Density Of A String
Mar 28, 2025
-
Which Type Of Substance Cannot Be Separated Physically
Mar 28, 2025
-
What Is The Transcribed Mrna Strand For Cattaa
Mar 28, 2025
-
What Is The Final Electron Acceptor In The Etc
Mar 28, 2025
-
The Purpose Of Cellular Respiration Is To
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Titanium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
