What Is The Electron Configuration Of Arsenic
News Leon
Mar 24, 2025 · 6 min read
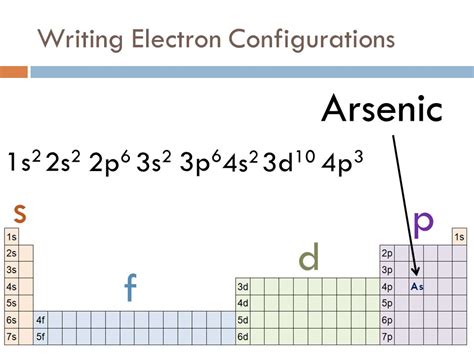
Table of Contents
What is the Electron Configuration of Arsenic? A Deep Dive into Atomic Structure
Arsenic, a metalloid with a fascinating array of properties and applications, holds a unique position on the periodic table. Understanding its electron configuration is key to unlocking its chemical behavior and explaining its diverse uses, from semiconductors to medicine (though its toxicity necessitates careful handling). This article will provide a comprehensive exploration of arsenic's electron configuration, delving into the underlying principles of atomic structure and the implications of this specific arrangement.
Understanding Electron Configuration
Before we dive into arsenic specifically, let's establish a foundational understanding of electron configuration. An electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. These arrangements are governed by the principles of quantum mechanics, which dictate that electrons occupy orbitals characterized by specific quantum numbers:
-
Principal Quantum Number (n): This number represents the energy level of the electron, with increasing values indicating higher energy levels (n = 1, 2, 3, etc.). Higher energy levels are further from the nucleus.
-
Azimuthal Quantum Number (l): This number defines the shape of the electron orbital and can have values from 0 to n-1. These values correspond to different sublevels:
- l = 0: s orbital (spherical)
- l = 1: p orbital (dumbbell-shaped)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. For a given value of l, ml can range from -l to +l, including 0.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, which can have a value of +1/2 or -1/2 (often represented as ↑ and ↓). The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons with opposite spins.
Arsenic's Position on the Periodic Table
Arsenic (As) is located in Group 15 (also known as Group VA or the pnictogens) and Period 4 of the periodic table. Its atomic number is 33, meaning it has 33 protons and, in its neutral state, 33 electrons. This positioning provides crucial clues about its electron configuration and chemical behavior. Group 15 elements are characterized by having five valence electrons (electrons in the outermost energy level), which significantly influence their reactivity.
Deriving Arsenic's Electron Configuration
To determine the electron configuration of arsenic, we follow the Aufbau principle, which states that electrons fill the lowest energy levels first. We also consider Hund's rule, which states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital.
The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p… and so on. However, exceptions exist, particularly with transition metals and some other elements.
Following this principle, the electron configuration of arsenic (As) is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p³.
Let's break this down:
- 1s²: Two electrons in the 1s orbital.
- 2s²: Two electrons in the 2s orbital.
- 2p⁶: Six electrons in the three 2p orbitals (two electrons per orbital).
- 3s²: Two electrons in the 3s orbital.
- 3p⁶: Six electrons in the three 3p orbitals.
- 4s²: Two electrons in the 4s orbital.
- 3d¹⁰: Ten electrons in the five 3d orbitals.
- 4p³: Three electrons in the three 4p orbitals (one electron in each orbital, following Hund's rule).
Noble Gas Configuration
A more concise way to represent arsenic's electron configuration is using the noble gas configuration. This involves using the symbol of the preceding noble gas (argon, Ar) to represent the filled inner shells: [Ar] 4s²3d¹⁰4p³. Argon's electron configuration is 1s²2s²2p⁶3s²3p⁶. This notation simplifies the representation while retaining all the essential information.
Valence Electrons and Chemical Reactivity
The valence electrons are the electrons in the outermost energy level, which are most involved in chemical bonding. In arsenic's case, these are the electrons in the 4s and 4p orbitals, totaling five electrons (4s²4p³). This explains arsenic's tendency to form compounds with oxidation states of +3 and +5, as it can either lose three electrons or five electrons to achieve a stable electron configuration.
Arsenic's chemistry is also influenced by its position in the p-block, meaning that its valence electrons are in p-orbitals, leading to more complex bonding scenarios compared to elements in the s-block.
Arsenic's Allotropes and Properties
The electron configuration impacts arsenic's physical properties. Arsenic exists in several allotropic forms, meaning it can exist in different structural modifications. These differences stem from how the arsenic atoms bond to each other, influenced by the arrangement of their valence electrons. The most common form is grey arsenic, a brittle, metallic-looking solid. The variations in allotropes influence properties like conductivity and reactivity.
Semiconductor Properties
The specific arrangement of electrons in arsenic's outermost shell (4s²4p³) contributes to its semiconducting properties. The availability of three unpaired electrons in the 4p subshell allows for the control of electrical conductivity, a key characteristic exploited in semiconductor technology. Doping arsenic into silicon, for example, is a common practice in creating n-type semiconductors.
Toxicity and Biological Roles
While arsenic is used in various applications, it's critically important to acknowledge its toxicity. This toxicity is intrinsically linked to its chemical reactivity and the potential for its compounds to interfere with biological processes. The ease with which arsenic can form bonds with other elements, particularly sulfur and oxygen, and its ability to substitute for phosphorus in biological molecules, contributes to its toxicity.
Arsenic in Applications
Despite its toxicity, arsenic finds uses in several applications:
- Semiconductors: As mentioned earlier, arsenic is crucial in semiconductor technology.
- Alloys: It can be added to lead-based alloys to enhance their properties.
- Pesticides: Historically, arsenic compounds were used as pesticides, although this practice is largely discontinued due to toxicity concerns.
- Wood Preservatives: Similarly, arsenic-based wood preservatives have been used, but safer alternatives are increasingly employed.
- Medicine (Historically): Certain arsenic compounds have been explored in the past for medicinal purposes, though their use is highly restricted due to toxicity.
Conclusion
The electron configuration of arsenic, [Ar] 4s²3d¹⁰4p³, is central to understanding its diverse properties and applications. This configuration determines its valence electron count, its ability to form various compounds, its semiconducting behavior, and its toxicity. While its use is regulated due to safety concerns, arsenic's unique atomic structure continues to be of scientific interest and practical importance in several technological fields. Further research continues to unravel the subtleties of arsenic's chemical behavior and its potential for future applications, always keeping in mind its inherent toxicity.
Latest Posts
Latest Posts
-
Is 536 Cm Bigger Than 53 6 Dm
Mar 26, 2025
-
How Much Hours Is 120 Minutes
Mar 26, 2025
-
What Number Is 45 Of 80
Mar 26, 2025
-
How Does An Aneroid Barometer Work
Mar 26, 2025
-
Why Political Parties Are Important For Democracy
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Arsenic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
