What Is The Difference Between Molecular Weight And Molar Mass
News Leon
Mar 25, 2025 · 5 min read
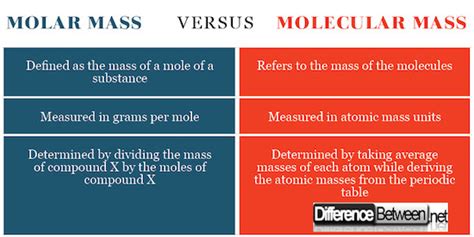
Table of Contents
- What Is The Difference Between Molecular Weight And Molar Mass
- Table of Contents
- What's the Difference Between Molecular Weight and Molar Mass?
- Understanding Molecular Weight
- Calculating Molecular Weight
- Units of Molecular Weight
- Limitations of Molecular Weight
- Delving into Molar Mass
- Calculating Molar Mass
- The Significance of Molar Mass
- Molar Mass of Elements and Compounds
- Molecular Weight vs. Molar Mass: A Clear Comparison
- When to Use Each Term
- Addressing Potential Confusion
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What's the Difference Between Molecular Weight and Molar Mass?
The terms "molecular weight" and "molar mass" are frequently used interchangeably, especially in informal settings. However, while closely related, they represent distinct concepts in chemistry. Understanding the subtle yet crucial differences is fundamental for accurate scientific communication and calculations. This article delves deep into the nuances of each term, clarifying their definitions, units, applications, and the contexts where their usage might be considered appropriate or inappropriate.
Understanding Molecular Weight
Molecular weight, also known as molecular mass, refers to the mass of a single molecule. It's calculated by summing the atomic weights of all the atoms present in a single molecule of a substance. The atomic weight of an element is the average mass of all its isotopes, weighted by their natural abundance. Therefore, molecular weight is an average value, reflecting the natural isotopic distribution of elements within the molecule.
Calculating Molecular Weight
The calculation is straightforward:
-
Identify the chemical formula: Determine the precise chemical formula of the molecule. For example, the chemical formula for water is H₂O.
-
Find atomic weights: Look up the atomic weights of each element in the periodic table. The atomic weight of hydrogen (H) is approximately 1.008 amu (atomic mass units), and the atomic weight of oxygen (O) is approximately 16.00 amu.
-
Calculate the molecular weight: Multiply the atomic weight of each element by the number of atoms of that element in the molecule and then sum the results. For water: (2 × 1.008 amu) + (1 × 16.00 amu) = 18.016 amu. Therefore, the molecular weight of water is approximately 18.016 amu.
Units of Molecular Weight
The standard unit for molecular weight is the atomic mass unit (amu), also known as the dalton (Da). Both units represent the mass of a single proton or neutron. While occasionally you might see molecular weight expressed in grams, it's important to understand this refers to the mass of a single molecule, not a mole of molecules.
Limitations of Molecular Weight
Molecular weight is inherently limited because it focuses on a single molecule. It doesn't directly provide information about the macroscopic properties of a substance, such as its mass in a given volume or the number of molecules present in a sample. This is where molar mass becomes indispensable.
Delving into Molar Mass
Molar mass represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance containing the same number of entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. This number, known as Avogadro's number, is approximately 6.022 × 10²³.
Calculating Molar Mass
The molar mass of a substance is numerically equivalent to its molecular weight, but the units are different. While molecular weight is expressed in amu, molar mass is expressed in grams per mole (g/mol). For water, the molar mass is 18.016 g/mol. This means that one mole of water molecules weighs 18.016 grams.
The Significance of Molar Mass
Molar mass is crucial for various chemical calculations, including:
-
Stoichiometry: Molar mass allows for the conversion between the mass of a substance and the number of moles, which is essential for balancing chemical equations and performing stoichiometric calculations.
-
Concentration calculations: Molarity (moles per liter) and molality (moles per kilogram of solvent) rely heavily on molar mass for determining the concentration of solutions.
-
Gas laws: The ideal gas law (PV = nRT) uses the number of moles (n), which is readily calculated using molar mass and the mass of the gas.
-
Thermochemistry: Molar mass is needed to determine the enthalpy, entropy, and Gibbs free energy changes in chemical reactions on a per-mole basis.
Molar Mass of Elements and Compounds
The molar mass concept applies equally to elements and compounds. For elements, the molar mass is simply the atomic weight expressed in grams per mole. For example, the molar mass of carbon (C) is 12.01 g/mol. For compounds, the molar mass is calculated by summing the molar masses of all the atoms in the chemical formula.
Molecular Weight vs. Molar Mass: A Clear Comparison
| Feature | Molecular Weight | Molar Mass |
|---|---|---|
| Definition | Mass of a single molecule | Mass of one mole of a substance |
| Unit | amu (atomic mass unit) or Dalton (Da) | g/mol (grams per mole) |
| Scale | Microscopic | Macroscopic |
| Application | Primarily for individual molecule properties | Essential for stoichiometry, concentration, gas laws, thermochemistry, etc. |
| Numerical Value | Numerically equivalent to molar mass | Numerically equivalent to molecular weight |
When to Use Each Term
While the numerical values are the same, the context dictates which term is more appropriate:
-
Use molecular weight when discussing the mass of a single molecule, or when dealing with mass spectrometry data, where individual molecule masses are measured. Generally, "molecular mass" is often used in this context.
-
Use molar mass in all macroscopic calculations involving moles, stoichiometry, and other quantitative aspects of chemistry. It's the preferred term in most formal scientific writing and chemical calculations.
Addressing Potential Confusion
The frequent interchangeability of the terms has led to some confusion. However, remembering the fundamental difference in units and the implications for scale of measurement provides clarity. Molecular weight focuses on the microscopic level (a single molecule), while molar mass operates on the macroscopic level (a mole of molecules).
Conclusion
Although often used interchangeably, molecular weight and molar mass represent distinct concepts with differing units and application domains. Molecular weight describes the mass of a single molecule in amu, while molar mass represents the mass of one mole of a substance in g/mol. While numerically equivalent, choosing the appropriate term ensures clear and accurate scientific communication. Understanding this distinction is vital for mastering fundamental chemistry principles and performing accurate chemical calculations. Maintaining this level of precision is essential for researchers, students, and anyone working within the field of chemistry. Understanding these subtle but important differences enhances your understanding of chemical principles and promotes precise scientific communication.
Latest Posts
Latest Posts
-
Which Of The Following Is Not An Example Of Matter
Mar 27, 2025
-
How Many Minutes In Two Hours
Mar 27, 2025
-
Oil And Water Is What Type Of Mixture
Mar 27, 2025
-
How Many Single Covalent Bonds Can A Carbon Atom Form
Mar 27, 2025
-
Which Of The Following Is The Most Stable Alkene
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Molecular Weight And Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
