What Is The Difference Between An Orbit And An Orbital
News Leon
Mar 18, 2025 · 6 min read
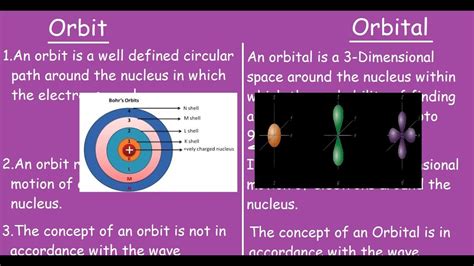
Table of Contents
What's the Difference Between an Orbit and an Orbital?
The terms "orbit" and "orbital" are frequently used in discussions about astronomy and quantum mechanics, but they represent fundamentally different concepts. While seemingly related due to their shared root, their meanings diverge significantly. Understanding this distinction is crucial for comprehending the intricacies of celestial mechanics and the quantum world.
Orbits: The Dance of Celestial Bodies
In astronomy and physics, an orbit is the path a celestial body takes around another celestial body due to gravitational attraction. This path can be elliptical, circular, parabolic, or hyperbolic, depending on the initial conditions and the strength of the gravitational forces involved.
Key Characteristics of Orbits:
-
Gravitational Interaction: Orbits are fundamentally defined by the gravitational interaction between two or more massive objects. The more massive object (like a star or planet) dominates the system, creating a gravitational well that the smaller object (like a planet or moon) follows.
-
Path Shape: The shape of the orbit is determined by the initial velocity and position of the orbiting object relative to the central object. A perfectly circular orbit requires a precise balance of speed and distance. Most orbits, however, are elliptical, with the central object located at one of the ellipse's foci. Parabolic and hyperbolic orbits are characteristic of objects that are not gravitationally bound to the central object and escape its influence.
-
Kepler's Laws: The motion of objects in orbits is governed by Kepler's three laws of planetary motion, which describe the relationship between the orbital period, distance, and the shapes of the orbits.
-
Orbital Elements: Precisely defining an orbit requires several parameters, known as orbital elements. These include the semi-major axis (average distance), eccentricity (deviation from a perfect circle), inclination (angle relative to a reference plane), longitude of the ascending node, argument of periapsis (closest approach), and mean anomaly (angular position at a specific time).
-
Examples of Orbits: Planets orbiting stars, moons orbiting planets, satellites orbiting Earth, and even binary stars orbiting each other are all examples of orbits.
Types of Orbits:
Understanding the various types of orbits is essential for mission planning, satellite deployments, and general celestial navigation. Here are some key categories:
-
Circular Orbits: These are idealized orbits where the orbiting body maintains a constant distance from the central body. While theoretically possible, they're rare in nature.
-
Elliptical Orbits: The most common type of orbit, characterized by variations in distance between the orbiting body and the central body. The closest point is called the periapsis (or perigee for Earth), and the farthest point is called the apoapsis (or apogee for Earth).
-
Parabolic Orbits: These orbits have an eccentricity of 1 and represent a single pass by the central body; the orbiting object will never return.
-
Hyperbolic Orbits: These orbits have an eccentricity greater than 1 and also represent a single pass, with the orbiting object escaping the gravitational influence of the central body at high speed.
-
Geostationary Orbits: A special type of geosynchronous orbit where a satellite appears stationary relative to a point on Earth's surface, crucial for communication and weather satellites.
-
Geosynchronous Orbits: Orbits where the satellite completes one revolution around the Earth in exactly one sidereal day.
-
Polar Orbits: Orbits inclined at nearly 90 degrees to the Earth's equator, often used for Earth observation satellites.
Orbitals: The Quantum Realm of Probability
In contrast to the well-defined paths of celestial bodies, an orbital in quantum mechanics describes a region of space around an atom's nucleus where there is a high probability of finding an electron. It's crucial to emphasize the probabilistic nature of orbitals; they don't represent fixed paths like orbits.
Key Differences from Orbits:
-
Probability, Not Path: Unlike orbits, which describe a specific path, orbitals describe a probability distribution. The orbital defines the region where an electron is most likely to be found, not where it is at any given moment.
-
Quantum Numbers: Each orbital is characterized by a set of quantum numbers (principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number) that determine its energy, shape, orientation, and electron spin.
-
Wave-Particle Duality: Electrons exhibit wave-particle duality; they behave as both waves and particles. Orbitals reflect this wave nature, describing the electron's wave function within the atom.
-
Shapes and Sizes: Orbitals have distinct shapes and sizes determined by their quantum numbers. The simplest orbital, the s orbital, is spherical; p orbitals are dumbbell-shaped; d orbitals are more complex. The size of an orbital is related to the principal quantum number; higher principal quantum numbers correspond to larger orbitals.
-
Electron Configuration: The arrangement of electrons in different orbitals within an atom is called its electron configuration. This configuration dictates the chemical properties of the atom.
Types of Orbitals:
-
s Orbitals: Spherical in shape, with one s orbital per energy level.
-
p Orbitals: Dumbbell-shaped, with three p orbitals (px, py, pz) oriented along the x, y, and z axes per energy level (starting from the second energy level).
-
d Orbitals: More complex shapes, with five d orbitals per energy level (starting from the third energy level).
-
f Orbitals: Even more complex shapes, with seven f orbitals per energy level (starting from the fourth energy level).
Understanding the Probability Density:
The probability of finding an electron at a particular point within an orbital is given by the square of the wave function (|Ψ|²). This probability density is highest at the center of the orbital and decreases as the distance from the nucleus increases. There's a non-zero probability of finding the electron at seemingly arbitrary distances, but the probability is exceedingly small beyond the typically depicted boundaries of the orbital.
Analogy to Help Understand the Difference:
Imagine a planet orbiting a star. The orbit is the precise path the planet follows around the star, predictable and repeatable. Now, imagine an electron in an atom. The orbital is more like a blurry cloud, representing the likelihood of finding the electron in various locations around the nucleus. You can't say the electron is traveling along a specific path within the cloud; instead, it has a certain probability of being at any point within the cloud's boundaries.
Summary Table: Orbit vs. Orbital
| Feature | Orbit | Orbital |
|---|---|---|
| System | Celestial Mechanics | Quantum Mechanics |
| Object | Celestial Body (planet, star, moon) | Electron |
| Definition | Path of a celestial body around another | Region of space with high electron probability |
| Nature | Deterministic, predictable | Probabilistic, uncertain |
| Governing Force | Gravity | Electromagnetic force (primarily) |
| Shape | Elliptical, circular, parabolic, hyperbolic | Spherical, dumbbell, cloverleaf, etc. |
| Description | Exact trajectory | Probability distribution |
Conclusion:
While both "orbit" and "orbital" involve movement around a central point, they represent drastically different concepts in vastly different realms of physics. Orbits describe the well-defined paths of celestial bodies under the influence of gravity, while orbitals describe the probabilistic locations of electrons within atoms governed by quantum mechanics. Understanding the distinctions between these terms is key to grasping the fundamentals of astronomy and quantum physics. The seemingly simple difference in terminology reflects a fundamental shift in how we understand the universe at macroscopic and microscopic scales. Appreciating this difference helps in a deeper understanding of both celestial mechanics and the quantum world, paving the way for further exploration and comprehension of the universe's intricate workings.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Form Of Precipitation
Mar 18, 2025
-
Which Statement About Natural Selection Is True
Mar 18, 2025
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between An Orbit And An Orbital . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
