What Is The Conjugate Base Of H2co3
News Leon
Mar 23, 2025 · 5 min read
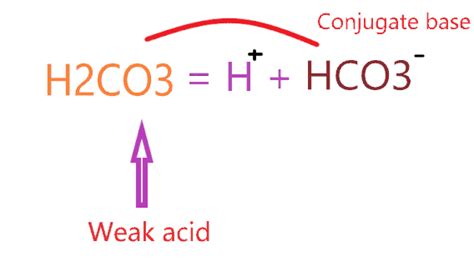
Table of Contents
- What Is The Conjugate Base Of H2co3
- Table of Contents
- What is the Conjugate Base of H₂CO₃? A Deep Dive into Carbonic Acid Chemistry
- Understanding Conjugate Acid-Base Pairs
- The Conjugate Bases of H₂CO₃: A Step-wise Breakdown
- The First Dissociation and the Bicarbonate Ion (HCO₃⁻)
- The Second Dissociation and the Carbonate Ion (CO₃²⁻)
- The Importance of Bicarbonate and Carbonate Ions
- 1. Blood pH Regulation: The Bicarbonate Buffer System
- 2. Marine Chemistry and Ocean Acidification
- 3. Industrial Applications
- Factors Affecting the Equilibrium
- Analytical Techniques for Studying Carbonic Acid and its Conjugate Bases
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What is the Conjugate Base of H₂CO₃? A Deep Dive into Carbonic Acid Chemistry
Carbonic acid (H₂CO₃) plays a vital role in various biological and chemical processes. Understanding its properties, particularly its conjugate bases, is crucial for comprehending these processes. This article provides a comprehensive exploration of H₂CO₃, focusing on its conjugate bases, their formation, and their significance in different contexts. We will delve into the intricacies of acid-base chemistry, exploring the equilibrium reactions and the implications of these reactions in various systems.
Understanding Conjugate Acid-Base Pairs
Before diving into the specifics of H₂CO₃, let's establish a fundamental understanding of conjugate acid-base pairs. According to the Brønsted-Lowry theory of acids and bases, an acid is a proton (H⁺) donor, and a base is a proton acceptor. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. The conjugate base always has one less proton than its corresponding acid.
In simpler terms: Imagine a tug-of-war between a proton and a molecule. The molecule holding onto the proton is the acid. When it lets go (donates the proton), the molecule remaining is its conjugate base.
The Conjugate Bases of H₂CO₃: A Step-wise Breakdown
Carbonic acid (H₂CO₃), a weak diprotic acid, can donate two protons. This means it has two conjugate bases. Let's examine each step:
The First Dissociation and the Bicarbonate Ion (HCO₃⁻)
The first dissociation of carbonic acid involves the loss of one proton:
H₂CO₃ ⇌ H⁺ + HCO₃⁻
In this reaction:
- H₂CO₃ (carbonic acid) acts as the acid, donating a proton.
- H⁺ (hydrogen ion or proton) is the donated proton.
- HCO₃⁻ (bicarbonate ion) is the conjugate base of H₂CO₃. It's formed after H₂CO₃ loses one proton.
The bicarbonate ion (HCO₃⁻) is an amphiprotic species, meaning it can act as both an acid and a base. It can further donate a proton, or it can accept a proton. Its ability to act as both an acid and a base is crucial in maintaining blood pH.
The Second Dissociation and the Carbonate Ion (CO₃²⁻)
The second dissociation of carbonic acid involves the bicarbonate ion (HCO₃⁻) losing its remaining proton:
HCO₃⁻ ⇌ H⁺ + CO₃²⁻
In this reaction:
- HCO₃⁻ (bicarbonate ion) acts as the acid, donating a proton.
- H⁺ (hydrogen ion or proton) is the donated proton.
- CO₃²⁻ (carbonate ion) is the conjugate base of HCO₃⁻, and thus the second conjugate base of H₂CO₃.
The Importance of Bicarbonate and Carbonate Ions
The conjugate bases of carbonic acid, bicarbonate (HCO₃⁻) and carbonate (CO₃²⁻), are incredibly important in various biological and chemical systems:
1. Blood pH Regulation: The Bicarbonate Buffer System
The bicarbonate buffer system is a crucial mechanism in maintaining the blood's pH within a narrow, life-sustaining range (around 7.4). This system involves the equilibrium between carbonic acid (H₂CO₃), bicarbonate (HCO₃⁻), and dissolved carbon dioxide (CO₂):
CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻
When the blood becomes too acidic (high H⁺ concentration), the equilibrium shifts to the left, consuming H⁺ ions and forming more H₂CO₃ and ultimately CO₂ which is exhaled. Conversely, if the blood becomes too alkaline (low H⁺ concentration), the equilibrium shifts to the right, producing more H⁺ ions. This delicate balance is essential for proper cellular function.
2. Marine Chemistry and Ocean Acidification
The carbonate ion (CO₃²⁻) is a major component of the ocean's buffering system. It reacts with dissolved CO₂ to form bicarbonate (HCO₃⁻):
CO₂ + CO₃²⁻ + H₂O ⇌ 2HCO₃⁻
However, increased atmospheric CO₂ levels due to human activities are leading to ocean acidification. This increased CO₂ dissolves in the ocean, forming more carbonic acid (H₂CO₃), which subsequently lowers the pH. The excess H⁺ ions react with carbonate ions (CO₃²⁻), reducing their concentration and affecting marine organisms that rely on carbonate for shell and skeleton formation (e.g., corals, shellfish).
3. Industrial Applications
Bicarbonate (HCO₃⁻) and carbonate (CO₃²⁻) have various industrial applications:
- Baking soda (sodium bicarbonate, NaHCO₃): Used as a leavening agent in baking.
- Antacids: Bicarbonate neutralizes stomach acid, relieving indigestion.
- Cement production: Carbonate-containing materials are crucial ingredients in cement manufacturing.
- Water treatment: Bicarbonate and carbonate ions are involved in water softening processes.
Factors Affecting the Equilibrium
Several factors influence the equilibrium reactions involving carbonic acid and its conjugate bases:
- Temperature: The equilibrium constants for the dissociation of carbonic acid are temperature-dependent.
- Pressure: Changes in pressure, particularly the partial pressure of CO₂, can shift the equilibrium.
- pH: The pH of the solution strongly affects the relative concentrations of H₂CO₃, HCO₃⁻, and CO₃²⁻.
- Concentration of reactants and products: The law of mass action dictates how changes in the concentrations of these species influence the equilibrium position.
Analytical Techniques for Studying Carbonic Acid and its Conjugate Bases
Various analytical techniques can be employed to study carbonic acid and its conjugate bases:
- Titration: Acid-base titrations can determine the concentration of H₂CO₃ and its conjugate bases in a solution.
- Spectroscopy: Spectroscopic methods, such as infrared (IR) and Raman spectroscopy, can be used to identify and quantify the different species.
- Electrochemical methods: Techniques like potentiometry can measure the pH and determine the concentrations of H⁺ ions, providing information about the equilibrium.
Conclusion
The conjugate bases of H₂CO₃, bicarbonate (HCO₃⁻) and carbonate (CO₃²⁻), are fundamental to various natural and industrial processes. Their role in maintaining blood pH, the complexities of ocean chemistry, and their numerous applications underscore their importance. Understanding the equilibrium reactions involving carbonic acid and its conjugate bases is crucial for comprehending diverse chemical and biological phenomena. Further research into these processes continues to reveal new insights into their profound impact on our environment and our lives. The interplay between carbonic acid, bicarbonate, and carbonate ions highlights the dynamic nature of chemical equilibrium and its significance in shaping our world. By appreciating the delicate balance of these species, we can better understand and address critical environmental challenges and develop innovative applications in various fields. From the human body to the vast expanse of the oceans, the chemistry of carbonic acid and its conjugate bases holds considerable weight and warrants continuous study and investigation.
Latest Posts
Latest Posts
-
Why Political Parties Are Important For Democracy
Mar 26, 2025
-
How To Calculate Long Run Equilibrium Price
Mar 26, 2025
-
Draw The Ozonolysis Products Of 2 Methyl 2 Pentene
Mar 26, 2025
-
The Correct Name For Feo Is
Mar 26, 2025
-
What Is The Distance Between Points A And B
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Base Of H2co3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
