What Is The Conjugate Acid Of Water
News Leon
Mar 22, 2025 · 6 min read
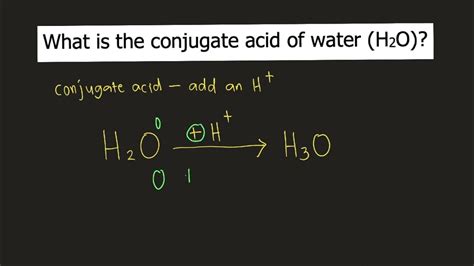
Table of Contents
What is the conjugate acid of water?
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This concept, central to Brønsted-Lowry acid-base theory, helps explain how acids and bases react with each other. This article delves deep into the concept of conjugate acid-base pairs, focusing specifically on the conjugate acid of water, providing a comprehensive understanding suitable for students and enthusiasts alike.
The Brønsted-Lowry Theory: A Foundation for Understanding Conjugate Pairs
Before we dive into the conjugate acid of water, it's crucial to establish the foundation of Brønsted-Lowry acid-base theory. This theory defines an acid as a substance that donates a proton (H⁺ ion), and a base as a substance that accepts a proton. Crucially, this theory emphasizes the transfer of a proton during an acid-base reaction.
This proton transfer leads to the formation of a conjugate acid-base pair. The conjugate acid is the species formed when a base accepts a proton, while the conjugate base is the species formed when an acid donates a proton. They are related by the difference of a single proton (H⁺).
In simpler terms: Imagine an acid losing a proton. What's left behind is its conjugate base. Conversely, when a base gains a proton, the resulting species is its conjugate acid.
Water: An Amphoteric Substance
Water (H₂O) possesses a unique property: it's amphoteric. This means water can act as both an acid and a base. This dual nature is pivotal in understanding its conjugate acid.
Water acting as an acid:
When water acts as an acid, it donates a proton to another substance (a base). This results in the formation of the hydroxide ion (OH⁻), which is the conjugate base of water.
H₂O ⇌ H⁺ + OH⁻
Water acting as a base:
When water acts as a base, it accepts a proton from another substance (an acid). This leads to the formation of the hydronium ion (H₃O⁺), which is the conjugate acid of water.
H₂O + H⁺ ⇌ H₃O⁺
The Conjugate Acid of Water: Hydronium Ion (H₃O⁺)
Therefore, the answer to the question, "What is the conjugate acid of water?" is unequivocally the hydronium ion (H₃O⁺). This positively charged ion is formed when a water molecule accepts a proton from an acid.
The formation of the hydronium ion is a critical process in numerous aqueous acid-base reactions. It's crucial to remember that the proton (H⁺) doesn't exist freely in aqueous solution; instead, it readily bonds with a water molecule to form the more stable hydronium ion. Many acid-base reactions are more accurately represented using the hydronium ion rather than the bare proton.
Understanding the Acid-Base Equilibrium of Water
The amphoteric nature of water also leads to an equilibrium reaction known as the autoionization of water:
2H₂O ⇌ H₃O⁺ + OH⁻
This equilibrium shows that water can react with itself to produce both hydronium and hydroxide ions. The equilibrium constant for this reaction is known as the ion product constant for water (Kw), which at 25°C is approximately 1.0 x 10⁻¹⁴.
This value is incredibly important because it illustrates that even in pure water, there are small, but significant, concentrations of both H₃O⁺ and OH⁻ ions. This autoionization is responsible for the inherent pH of pure water, which is 7 at 25°C (neutral pH).
Examples of Reactions Involving the Hydronium Ion
The hydronium ion is a central player in numerous acid-base reactions. Let's examine a few key examples:
1. Reaction of a strong acid with water:
Consider the reaction of hydrochloric acid (HCl), a strong acid, with water:
HCl + H₂O → H₃O⁺ + Cl⁻
In this reaction, HCl acts as a proton donor (acid), donating a proton to water, which acts as a proton acceptor (base). The resulting products are the hydronium ion and the chloride ion (Cl⁻), the conjugate base of HCl. The reaction goes essentially to completion because HCl is a strong acid.
2. Reaction of a weak acid with water:
Let's examine the reaction of acetic acid (CH₃COOH), a weak acid, with water:
CH₃COOH + H₂O ⇌ H₃O⁺ + CH₃COO⁻
Unlike the strong acid example, this reaction is an equilibrium. Acetic acid only partially dissociates in water, meaning a significant amount of undissociated acetic acid remains. The equilibrium constant for this reaction (Ka) reflects the extent of dissociation. The hydronium ion is again a product of the reaction.
3. Neutralization reactions:
Neutralization reactions, where an acid and a base react to form salt and water, frequently involve the formation and consumption of hydronium ions. For example, the reaction between hydrochloric acid and sodium hydroxide:
HCl + NaOH → NaCl + H₂O
While this equation doesn't explicitly show the hydronium ion, it's crucial to understand that the HCl is actually reacting as H₃O⁺ in the aqueous solution.
The Importance of the Hydronium Ion in pH Calculations
The concentration of hydronium ions (H₃O⁺) directly determines the pH of a solution. The pH is defined as the negative logarithm (base 10) of the hydronium ion concentration:
pH = -log₁₀[H₃O⁺]
A lower pH indicates a higher concentration of hydronium ions and a more acidic solution. Conversely, a higher pH indicates a lower concentration of hydronium ions and a more basic solution. Accurate pH measurements are essential in numerous applications, including environmental monitoring, chemical analysis, and biological research, and all rely on the understanding of hydronium ion concentration.
Distinguishing between H⁺ and H₃O⁺
It's crucial to clarify the distinction between the proton (H⁺) and the hydronium ion (H₃O⁺). While textbooks often use H⁺ for simplicity, it's more accurate to use H₃O⁺ in aqueous solutions. A bare proton, due to its high charge density, is highly reactive and doesn't exist independently in water. It immediately interacts with a water molecule, forming the more stable hydronium ion.
Therefore, while both notations represent the acidic nature of the solution, using H₃O⁺ provides a more accurate representation of the chemical species present in aqueous solutions.
Beyond Water: Conjugate Acids of Other Bases
The concept of conjugate acids isn't limited to water. Many other bases have conjugate acids. For example:
- Ammonia (NH₃): The conjugate acid of ammonia is the ammonium ion (NH₄⁺).
- Bicarbonate ion (HCO₃⁻): The conjugate acid of the bicarbonate ion is carbonic acid (H₂CO₃).
- Sulfate ion (SO₄²⁻): The conjugate acid of the sulfate ion is the bisulfate ion (HSO₄⁻).
Understanding this concept broadly allows for a deeper grasp of acid-base chemistry in various contexts.
Conclusion: The Hydronium Ion - A Cornerstone of Acid-Base Chemistry
The hydronium ion (H₃O⁺), the conjugate acid of water, plays a crucial role in understanding acid-base reactions in aqueous solutions. Its presence significantly impacts pH, equilibrium reactions, and the overall behavior of acids and bases in water. Grasping the concept of conjugate acid-base pairs, along with the unique amphoteric nature of water, is essential for a solid foundation in chemistry. By recognizing the central role of the hydronium ion, we gain a deeper appreciation for the dynamic interactions that occur in aqueous acid-base systems, allowing for more accurate predictions and interpretations of experimental data. This knowledge extends beyond classroom studies, impacting diverse fields from environmental science to medicine and industrial processes. Further exploration into the intricacies of acid-base chemistry, particularly concerning the role of the hydronium ion, will continue to unlock new understandings and advancements in various scientific disciplines.
Latest Posts
Latest Posts
-
What Typ Of Ions Do Acids Release
Mar 24, 2025
-
Differences Among Individuals Of A Species Are Referred To As
Mar 24, 2025
-
What Is 77 F In Celsius
Mar 24, 2025
-
The Spinning Of Earth On Its Axis Is Called
Mar 24, 2025
-
Abo Blood Grouping Is An Example Of
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
