What Is The Charge Of Fe
News Leon
Mar 18, 2025 · 6 min read
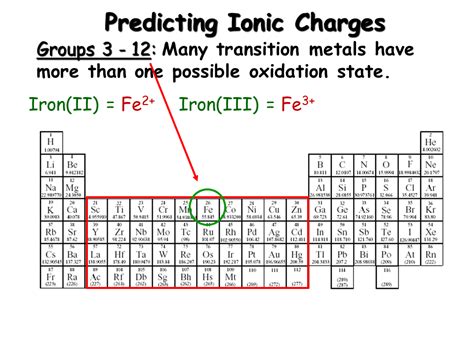
Table of Contents
What is the Charge of Fe? Understanding Iron's Oxidation States and Their Significance
Iron (Fe), a ubiquitous element essential for life and industry, doesn't possess a single, fixed charge. Instead, its charge, more accurately described as its oxidation state, varies depending on its chemical environment and bonding partners. This variable charge is a key factor in iron's diverse chemistry and its crucial roles in biological and industrial processes. Understanding the different oxidation states of iron is crucial for comprehending its reactivity, applications, and significance.
What is Oxidation State?
Before diving into the specific oxidation states of iron, it's vital to grasp the concept of oxidation state itself. Oxidation state, also known as oxidation number, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were completely ionic. It's a bookkeeping tool that helps us understand electron transfer and the reactivity of elements in compounds. It's important to remember that oxidation states are not necessarily the true charges on atoms, especially in covalent compounds where electrons are shared, not fully transferred.
Common Oxidation States of Iron
Iron exhibits several oxidation states, but the most common are +2 (ferrous) and +3 (ferric). Let's delve deeper into each:
1. Fe²⁺ (Ferrous):
The ferrous ion, Fe²⁺, is iron in its +2 oxidation state. This means it has lost two electrons compared to its neutral atomic state. Compounds containing Fe²⁺ are often referred to as ferrous compounds. Some examples include:
- Iron(II) sulfate (FeSO₄): A common iron supplement and also used in water treatment.
- Iron(II) oxide (FeO): A black, crystalline solid used in the production of iron and steel.
- Iron(II) chloride (FeCl₂): Used as a reducing agent and in various industrial processes.
Properties of Fe²⁺ compounds: Ferrous compounds are generally less stable than ferric compounds and readily oxidize to the +3 state in the presence of oxygen. They often exhibit a pale green color in solution.
2. Fe³⁺ (Ferric):
The ferric ion, Fe³⁺, represents iron in its +3 oxidation state. It has lost three electrons compared to its neutral state. Compounds containing Fe³⁺ are known as ferric compounds. Examples include:
- Iron(III) oxide (Fe₂O₃): The main component of rust and also used as a pigment (rust-red). This is a very common and stable form of iron.
- Iron(III) chloride (FeCl₃): A strong oxidizing agent used as a catalyst and in water treatment.
- Iron(III) hydroxide (Fe(OH)₃): A gelatinous precipitate that forms when a base is added to a solution containing Fe³⁺.
Properties of Fe³⁺ compounds: Ferric compounds are generally more stable than ferrous compounds, particularly in oxidizing environments. They often exhibit a yellowish-brown or reddish-brown color in solution.
Less Common Oxidation States:
While +2 and +3 are the most prevalent, iron can also exhibit other, less common oxidation states, including:
- +1 (Fe⁺): Relatively unstable and less frequently encountered.
- +4 (Fe⁴⁺): Extremely rare and usually found in highly specific and often unstable compounds.
- +5, +6: These are even rarer and typically found in very specialized, high-oxidation state compounds under very specific conditions.
- 0: Elemental iron, in its metallic form, has an oxidation state of 0.
Factors Influencing Iron's Oxidation State
Several factors determine which oxidation state iron adopts in a particular compound or situation:
- The presence of oxidizing or reducing agents: Oxidizing agents (like oxygen) tend to favor the +3 state, while reducing agents can stabilize the +2 state.
- pH: The acidity or alkalinity of the environment plays a significant role. The +3 state is often more stable in acidic conditions, while the +2 state can be favored in alkaline conditions.
- Ligands: The ligands (atoms, ions, or molecules bound to the central iron atom) can significantly influence the stability of different oxidation states. Certain ligands preferentially stabilize Fe²⁺, while others favor Fe³⁺.
- Temperature and pressure: These factors can also influence the stability of different oxidation states, although their impact is often less dramatic than the others.
Significance of Iron's Variable Oxidation States
The ability of iron to exist in multiple oxidation states is crucial for its diverse applications and biological roles:
Biological Significance:
- Hemoglobin and Myoglobin: Iron in the +2 state is essential for oxygen transport in hemoglobin (in red blood cells) and oxygen storage in myoglobin (in muscle tissue). The iron atom in the heme group undergoes reversible oxidation-reduction reactions, enabling oxygen binding and release.
- Cytochromes: Iron plays a vital role in the electron transport chain in cellular respiration, where it cycles between the +2 and +3 oxidation states, facilitating electron transfer.
- Enzymes: Many enzymes, like catalase and peroxidase, require iron as a cofactor to catalyze crucial biochemical reactions.
Industrial Applications:
- Steel Production: The production of steel relies heavily on the reduction of iron oxides (Fe₂O₃ and Fe₃O₄) to elemental iron (Fe) in a blast furnace.
- Catalysis: Iron compounds are used as catalysts in various industrial processes, such as ammonia synthesis (Haber-Bosch process) and the production of chemicals.
- Pigments: Iron oxides are used as pigments in paints, inks, and cosmetics, providing a wide range of colors depending on the oxidation state and crystal structure.
- Batteries: Iron-based batteries are being developed as cost-effective and environmentally friendly alternatives to lithium-ion batteries.
Determining the Oxidation State of Iron in Compounds
Determining the oxidation state of iron in a compound involves a systematic approach:
-
Assign oxidation states to other elements: Start by assigning oxidation states to elements with known oxidation states, such as oxygen (-2), hydrogen (+1), and alkali metals (+1).
-
Use the overall charge of the compound: The sum of the oxidation states of all atoms in a neutral compound must equal zero. For ions, the sum must equal the charge of the ion.
-
Solve for the oxidation state of iron: Use the known oxidation states of other elements and the overall charge to calculate the oxidation state of iron.
Example: Let's determine the oxidation state of iron in Fe₂O₃.
- Oxygen has an oxidation state of -2.
- There are three oxygen atoms, giving a total negative charge of 3 * (-2) = -6.
- The overall charge of the compound is 0.
- Let x be the oxidation state of iron. We have 2x + (-6) = 0.
- Solving for x, we get 2x = +6, so x = +3. Therefore, the oxidation state of iron in Fe₂O₃ is +3.
Conclusion
The charge, or more accurately the oxidation state, of iron is not a fixed value but rather a variable property dependent on its chemical environment. The most common oxidation states are +2 (ferrous) and +3 (ferric), each with distinct properties and significant roles in biological and industrial processes. Understanding the factors that influence iron's oxidation state and the methods for determining it is crucial for comprehending its diverse chemistry and wide-ranging applications. The versatility of iron, dictated by its variable oxidation states, highlights its importance in various fields, underscoring its continued relevance in scientific research and technological advancements. Further research into the less common oxidation states continues to uncover new applications and insights into this fundamental element.
Latest Posts
Latest Posts
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
-
How Many Water Molecules In A Drop Of Water
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of Fe . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
