What Is The Atomic Number Of An Atom Equivalent To
News Leon
Mar 23, 2025 · 6 min read
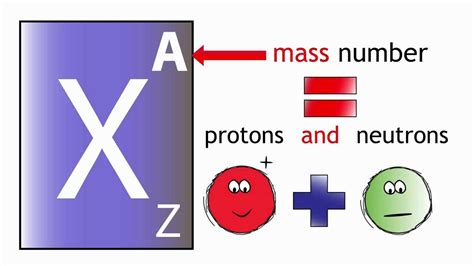
Table of Contents
What is the Atomic Number of an Atom Equivalent To?
The atomic number of an atom is a fundamental property that defines its identity and chemical behavior. It's more than just a number; it's a key that unlocks a wealth of information about an element's properties and its place within the periodic table. Understanding what the atomic number is equivalent to is crucial to grasping the very essence of chemistry and the structure of matter. This article will delve deep into the meaning of atomic number, exploring its equivalence to the number of protons, its role in defining elements, its connection to isotopes, and its implications for chemical reactivity and other atomic properties.
The Atomic Number: A Defining Characteristic
The atomic number of an atom is equivalent to the number of protons found in its nucleus. This seemingly simple statement holds profound implications. The nucleus, residing at the atom's center, contains positively charged protons and electrically neutral neutrons. Electrons, negatively charged particles, orbit the nucleus in shells or energy levels. While neutrons contribute to an atom's mass, it's the number of protons that uniquely identifies the element.
Why Protons Matter More Than Neutrons
Neutrons, despite contributing to an atom's mass, do not determine its elemental identity. Atoms of the same element can have varying numbers of neutrons, leading to the existence of isotopes. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. For example, carbon-12 and carbon-14 are both isotopes of carbon; they both have 6 protons, but carbon-12 has 6 neutrons, while carbon-14 has 8. The atomic number remains constant (6 for carbon) irrespective of the number of neutrons.
It is the proton count that fundamentally dictates an atom's chemical behavior. The number of protons determines the number of electrons required to achieve electrical neutrality. This electron configuration, in turn, directly influences the atom's ability to form chemical bonds with other atoms, defining its reactivity and its place in the periodic table.
The Atomic Number and the Periodic Table
The periodic table, a cornerstone of chemistry, is organized based on atomic numbers. Elements are arranged in increasing order of their atomic number, reflecting their fundamental properties. The periodic table's structure is not arbitrary; it's a direct consequence of the periodic repetition of electron shell configurations, which themselves stem from the increasing number of protons. This arrangement allows for the prediction of chemical and physical properties based solely on an element's position within the table.
Understanding Periodic Trends
The atomic number's role in determining the periodic trends in properties such as electronegativity, ionization energy, and atomic radius is crucial. Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period (from left to right) as the nuclear charge increases (more protons). Ionization energy, the energy required to remove an electron from an atom, also increases across a period due to the increased attractive force of the nucleus. Atomic radius, the size of an atom, generally decreases across a period due to the increased nuclear charge pulling the electrons closer to the nucleus.
These trends, easily visualized on the periodic table, highlight the powerful link between atomic number and the observable properties of elements. The atomic number serves as the foundation upon which our understanding of these predictable patterns is built.
Isotopes and Atomic Number: A Deeper Dive
As previously mentioned, isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. While the atomic number remains consistent for all isotopes of a given element, the mass number (the sum of protons and neutrons) varies. This difference in mass number leads to variations in the physical properties of isotopes, such as their mass and radioactive decay behavior.
Radioactive Isotopes and their Applications
Some isotopes are radioactive, meaning their nuclei are unstable and undergo decay, emitting particles or energy. Radioactive isotopes, identified by their atomic number and mass number, have numerous applications in various fields, including medicine (radiotherapy, medical imaging), archaeology (radiocarbon dating), and industrial processes (gauging, sterilization). The understanding and application of radioactive isotopes hinge upon the fundamental concept of atomic number, which identifies the element involved in the decay process.
Atomic Number and Chemical Reactivity
The atomic number dictates the electron configuration of an atom, which is the key determinant of its chemical reactivity. The outermost electrons, called valence electrons, are particularly important because they participate in chemical bonding. The number of valence electrons is directly related to the atomic number and determines an element's ability to form covalent bonds (sharing electrons), ionic bonds (transferring electrons), or metallic bonds.
Valence Electrons and Chemical Bonding
Atoms tend to react in ways that allow them to achieve a stable electron configuration, often resembling that of a noble gas (group 18 elements). This stable configuration, usually involving a full outer electron shell, drives chemical reactions and determines the types of chemical bonds that can be formed. The number of valence electrons, indirectly determined by the atomic number, directly impacts the atom's reactivity and the types of compounds it can form.
Beyond the Basics: Advanced Concepts
The concept of atomic number extends beyond basic chemical behavior to more advanced areas of chemistry and physics. Nuclear chemistry, for example, extensively uses atomic number to understand nuclear reactions and radioactive decay. Nuclear fission and fusion, processes involving changes in the nucleus, are deeply intertwined with atomic number and mass number. Furthermore, the atomic number plays a crucial role in understanding the behavior of elements in extreme conditions, such as those found in stars or in high-energy particle accelerators.
Atomic Number in Nuclear Physics
In nuclear physics, the atomic number is crucial for identifying the elements involved in nuclear reactions. Nuclear reactions, unlike chemical reactions, involve changes in the nucleus, affecting the number of protons and neutrons. These changes can lead to the transformation of one element into another, a process called transmutation. The understanding of nuclear reactions, including nuclear fission and fusion, relies heavily on the precise identification of atomic numbers of the elements involved.
Conclusion: The Power of the Atomic Number
The atomic number, seemingly a simple numerical value, is fundamentally equivalent to the number of protons in an atom's nucleus. This simple equivalence unlocks a vast understanding of an atom's identity, chemical behavior, and position within the periodic table. From determining chemical reactivity to understanding nuclear processes, the atomic number serves as a cornerstone of our understanding of matter and its interactions. It's a testament to the power of fundamental concepts in unlocking the complexities of the natural world. Its significance spans across various fields, from basic chemistry to advanced nuclear physics, highlighting its fundamental role in our understanding of the universe at its most fundamental level. The atomic number is not merely a number; it is a defining characteristic that holds the key to unlocking the secrets of the elements.
Latest Posts
Latest Posts
-
Which Of The Following Are Not Polynomials
Mar 24, 2025
-
The Stratum Lucidum Is Found Only In
Mar 24, 2025
-
The Splitting Apart Of Atomic Nuclei Is Known As
Mar 24, 2025
-
What Is The Molar Mass Of Bf3
Mar 24, 2025
-
Which Of The Following Would Not Be A Physical Change
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Is The Atomic Number Of An Atom Equivalent To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
