What Is The Approximate Mass Of One Proton
News Leon
Mar 30, 2025 · 6 min read
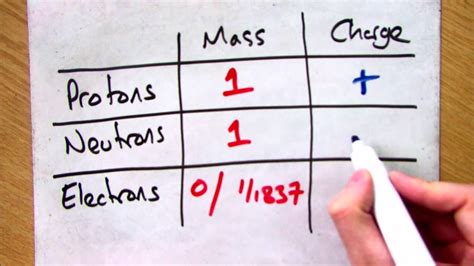
Table of Contents
What is the Approximate Mass of One Proton? Delving into the Subatomic World
The seemingly simple question, "What is the approximate mass of one proton?" opens a fascinating window into the realm of particle physics and the fundamental building blocks of matter. While the answer itself might seem straightforward, understanding its implications requires a deeper dive into the intricacies of atomic structure, measurement techniques, and the units we use to quantify such incredibly small quantities.
Understanding Protons: The Heart of the Atom
Before we delve into the mass, let's establish a clear understanding of what a proton actually is. Protons are subatomic particles found within the nucleus of an atom. They carry a positive electrical charge, equal in magnitude but opposite in sign to the charge of an electron. Crucially, the number of protons in an atom's nucleus defines the element itself; an atom with one proton is hydrogen, two protons is helium, and so on. This fundamental property directly influences the atom's chemical behavior and properties.
Protons, along with neutrons (which have no charge), make up the bulk of an atom's mass. Electrons, while contributing to the atom's overall charge and chemical reactivity, are significantly less massive. This concentration of mass in the nucleus is a cornerstone of atomic theory.
Measuring the Mass of a Proton: A Tale of Precision
Measuring the mass of a single proton presents a significant challenge. We're dealing with an incredibly tiny particle, far smaller than anything visible with even the most powerful optical microscopes. The techniques used to determine this mass are sophisticated and rely on advanced physics principles.
One primary method involves utilizing mass spectrometry. This technique involves ionizing atoms, accelerating them through electric and magnetic fields, and then measuring their deflection based on their mass-to-charge ratio. By carefully calibrating the instrument and analyzing the deflection patterns, scientists can deduce the mass of the proton with high accuracy.
Another technique involves using particle accelerators, such as the Large Hadron Collider (LHC). These powerful machines accelerate particles to extremely high energies, allowing scientists to study their interactions and properties with unprecedented precision. By analyzing the products of collisions involving protons, researchers can indirectly deduce their mass with remarkable accuracy.
These methods, along with other sophisticated techniques, contribute to an increasingly precise understanding of the proton's mass.
Expressing the Mass: Units and Conventions
The mass of a proton is typically expressed using several different units, each with its own advantages and applications. The most commonly used units are:
-
Atomic Mass Units (amu or u): This unit is defined relative to the mass of a carbon-12 atom, with one amu being exactly 1/12 the mass of a carbon-12 atom. It's particularly convenient in chemistry and related fields because it directly relates to the relative masses of atoms and isotopes. In amu, the mass of a proton is approximately 1.007276 amu.
-
Kilograms (kg): This is the standard unit of mass in the International System of Units (SI). While seemingly less intuitive for subatomic particles, using kilograms allows for consistent calculations across different fields of physics. The mass of a proton in kilograms is approximately 1.67262 × 10^-27 kg. This extremely small number highlights the minuscule scale we're dealing with.
-
Electronvolts (eV): This unit is commonly used in particle physics and expresses energy. Through Einstein's famous equation, E=mc², mass and energy are directly related. Therefore, the mass of a proton can also be expressed in energy units. The proton's mass is approximately 938.27 MeV/c², where MeV stands for mega-electronvolts and c represents the speed of light. This representation is particularly useful when discussing high-energy particle interactions.
The Approximate Mass: A Practical Perspective
Given the various units and the high precision of modern measurement techniques, it's helpful to establish a convenient "approximate" mass for practical purposes. For many applications, particularly in introductory chemistry and physics, the following approximations are commonly used:
- 1 amu (Atomic Mass Unit)
- 1.67 x 10^-27 kg (Kilograms)
These approximations are sufficiently accurate for many calculations and help simplify problem-solving without sacrificing significant accuracy. However, it's crucial to remember that these are simplifications and the precise value is crucial for advanced calculations and research.
Beyond the Simple Number: The Intricacies of Proton Mass
The mass of a proton isn't simply a fixed number; its understanding is intertwined with a deeper exploration of quantum chromodynamics (QCD), the theory that describes the strong nuclear force. The strong force is responsible for binding quarks together to form protons and neutrons.
Protons are composed of three quarks: two up quarks and one down quark. However, the mass of these constituent quarks only accounts for a small fraction of the proton's total mass. A significant portion comes from the energy associated with the strong force binding these quarks together, as well as the kinetic energy of the quarks and gluons (the particles mediating the strong interaction). This "extra" mass is a manifestation of Einstein's mass-energy equivalence (E=mc²).
Understanding this complex interplay between quarks, gluons, and the strong force is a central challenge in modern physics, and ongoing research continues to refine our understanding of the proton's mass and its underlying structure.
The Importance of Precision: Applications of Proton Mass Knowledge
Precise knowledge of the proton's mass is not merely an academic pursuit; it has significant practical implications across diverse fields:
-
Nuclear Physics: Accurate mass measurements are fundamental to understanding nuclear reactions, stability, and decay processes. This is crucial for applications such as nuclear energy, medicine (radioactive isotopes), and material science.
-
Particle Physics: The proton's mass plays a critical role in models of the universe's evolution and the behavior of elementary particles. Precise mass determination helps to test and refine our understanding of fundamental forces and interactions.
-
Cosmology: Understanding the masses of fundamental particles, including protons, is essential for building accurate models of the early universe and its subsequent evolution. This impacts our understanding of processes like nucleosynthesis (the formation of atomic nuclei in the early universe).
-
Chemistry and Materials Science: Accurate knowledge of atomic masses, which are directly tied to the number of protons, is crucial for determining chemical properties, reaction rates, and the behavior of materials.
Conclusion: A Journey into the Heart of Matter
The seemingly straightforward question of the proton's approximate mass leads us on a journey through the intricate world of subatomic particles, sophisticated measurement techniques, and profound theoretical concepts. While a simplified answer might suffice for many applications, a deeper understanding of the proton's mass, its constituent components, and its connection to fundamental forces is essential for advancing our knowledge of the universe and its building blocks. The ongoing research and refinement of our understanding continue to shape our understanding of physics and its numerous applications across diverse scientific disciplines. From the simplest chemical reaction to the most complex cosmological model, the proton's mass plays a fundamental role in shaping our world.
Latest Posts
Latest Posts
-
State Any Two Effects Of Force
Apr 01, 2025
-
How Many Electrons Does Sodium Have In Its Outer Shell
Apr 01, 2025
-
Dense Irregular Connective Tissue Will Be Found In The
Apr 01, 2025
-
Which Of The Following Organisms Can Fix Nitrogen
Apr 01, 2025
-
Which Of The Following Is Correct About Viruses
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Approximate Mass Of One Proton . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
