What Is Pseudo First Order Reaction
News Leon
Mar 23, 2025 · 6 min read
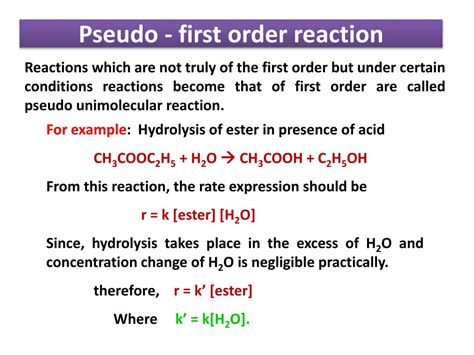
Table of Contents
What is a Pseudo First-Order Reaction? A Comprehensive Guide
Understanding reaction kinetics is crucial in chemistry, particularly when predicting the rates of chemical processes. While many reactions follow straightforward rate laws, others exhibit more complex behavior. One such case is the pseudo first-order reaction, a seemingly paradoxical concept that simplifies the analysis of complex reactions. This article will delve deep into the intricacies of pseudo first-order reactions, exploring their definition, mechanisms, applications, and significance in various fields.
Defining Pseudo First-Order Reactions
A pseudo first-order reaction is a chemical reaction that appears to follow first-order kinetics, even though it's actually a higher-order reaction. This deceptive simplicity arises when one of the reactants is present in significant excess compared to the others. The concentration of the reactant in excess remains essentially constant throughout the reaction, even as the other reactants are consumed. Consequently, the rate of the reaction becomes dependent only on the concentration of the limiting reactant, mimicking a first-order reaction.
In simpler terms: Imagine a reaction involving two reactants, A and B. If the concentration of B is vastly larger than A, the change in [B] during the reaction is negligible. The rate equation, which might inherently be second-order (or higher), effectively reduces to a first-order expression dependent solely on [A].
Understanding the Mechanism: Why the Simplification Works
The key to understanding pseudo first-order reactions lies in the rate law. Let's consider a generic second-order reaction:
A + B → Products
The rate law for this reaction is typically:
Rate = k[A][B]
Where:
- k is the rate constant
- [A] and [B] are the concentrations of reactants A and B, respectively.
Now, if we have a significant excess of B ([B] >> [A]), the concentration of B changes so little during the reaction that it can be considered constant. We can then incorporate [B] into the rate constant, creating a new "pseudo" rate constant, k':
Rate = k[A][B] ≈ k'[A] where k' = k[B]
This modified rate equation now resembles a first-order reaction, even though the underlying reaction mechanism is second-order. This is the essence of a pseudo first-order reaction: the apparent simplification of a higher-order reaction due to an overwhelming excess of one reactant.
Examples of Pseudo First-Order Reactions
Numerous chemical reactions exhibit pseudo first-order kinetics under specific conditions. Here are a few illustrative examples:
1. Acid-Catalyzed Hydrolysis of Esters:
The hydrolysis of an ester in the presence of a strong acid is a classic example. The reaction is typically second-order, involving both the ester and water. However, if the reaction is carried out in dilute aqueous solution, the concentration of water remains essentially constant throughout the reaction. This makes the reaction appear first-order with respect to the ester concentration only.
2. Inversion of Sucrose:
The acid-catalyzed inversion of sucrose into glucose and fructose is another well-known example. The reaction is second-order, but in the presence of a large excess of acid, it behaves as a pseudo first-order reaction with respect to sucrose.
3. Enzyme-Catalyzed Reactions:
Many enzyme-catalyzed reactions exhibit pseudo first-order kinetics under certain conditions. If the substrate concentration is much higher than the enzyme concentration, the enzyme is saturated with substrate, and the reaction rate becomes dependent primarily on the enzyme concentration. This can result in pseudo first-order kinetics with respect to the substrate.
Experimental Determination of Pseudo First-Order Rate Constants
Determining the rate constant for a pseudo first-order reaction involves similar techniques as for true first-order reactions. The most common method is to measure the concentration of the limiting reactant at various time intervals and then plot the data appropriately.
1. Integrated Rate Law:
For a pseudo first-order reaction, the integrated rate law is analogous to that of a true first-order reaction:
ln([A]t) = ln([A]0) - k't
Where:
- [A]t is the concentration of reactant A at time t
- [A]0 is the initial concentration of reactant A
- k' is the pseudo first-order rate constant
- t is the time
A plot of ln([A]t) versus t will yield a straight line with a slope of -k' and a y-intercept of ln([A]0).
2. Half-Life:
The half-life (t1/2) of a pseudo first-order reaction is also similar to that of a first-order reaction:
t1/2 = 0.693 / k'
The half-life remains constant throughout the reaction, unlike in higher-order reactions.
Applications of Pseudo First-Order Reactions
The concept of pseudo first-order reactions is not just a theoretical curiosity; it has significant practical applications across various fields:
1. Pharmaceutical Kinetics:
In drug metabolism, many reactions involving drug breakdown follow pseudo first-order kinetics. This simplification allows researchers to model drug elimination and determine factors like half-life and clearance rate, vital in dosage adjustments and drug design.
2. Environmental Chemistry:
The degradation of pollutants in the environment can often be described using pseudo first-order models. Factors like sunlight, soil composition, and microbial activity can influence the rate constant and provide insights into environmental remediation strategies.
3. Food Science:
The shelf life of many food products is governed by the kinetics of spoilage reactions. Modeling these reactions as pseudo first-order can assist in predicting shelf life and optimizing storage conditions.
4. Chemical Engineering:
In reactor design and optimization, understanding reaction kinetics is paramount. Using pseudo first-order approximations simplifies reactor design calculations, improving efficiency and cost-effectiveness.
Distinguishing Pseudo First-Order from True First-Order Reactions
It is crucial to recognize the difference between a true first-order reaction and a pseudo first-order reaction. The key lies in the underlying reaction mechanism and the dependence on reactant concentrations.
A true first-order reaction inherently depends only on the concentration of one reactant, regardless of the concentrations of other reactants. A pseudo first-order reaction, however, is fundamentally a higher-order reaction that exhibits first-order behavior due to a significant excess of one reactant.
Determining if a reaction is truly first-order or pseudo first-order requires careful experimental design. By systematically varying the concentrations of all reactants and observing the effect on the reaction rate, one can unveil the true order of the reaction.
Limitations and Considerations
While the pseudo first-order approximation simplifies analysis, it does have limitations:
-
Accuracy: The approximation works best when the excess reactant is truly in vast excess. As the concentration of the excess reactant decreases significantly, the deviation from first-order behavior increases, affecting the accuracy of calculations.
-
Reaction Mechanism: The simplification masks the true complexity of the reaction mechanism. Detailed mechanistic studies might require abandoning the pseudo first-order approximation.
-
Applicability: The approximation is not universally applicable. It only works for reactions where one reactant is significantly more abundant than others.
Conclusion
Pseudo first-order reactions are a valuable tool in chemical kinetics. This simplification of complex rate laws allows for easier analysis and modeling of reaction rates, even if the underlying mechanism is more intricate. Understanding its application and limitations is vital for researchers and engineers across various scientific and industrial fields. The ability to recognize and utilize pseudo first-order kinetics represents a critical skill in mastering reaction kinetics and its widespread applications. While it offers considerable simplification, it's crucial to remember that it’s an approximation and its accuracy is contingent upon maintaining a significant excess of one reactant throughout the reaction. Careful experimental design and awareness of these limitations are crucial for its effective application.
Latest Posts
Latest Posts
-
As The Frequency Of A Wave Increases The Wavelength
Mar 25, 2025
-
Simplest Rationalising Factor Of Root 50
Mar 25, 2025
-
The Red Data Book Keeps A Record Of All The
Mar 25, 2025
-
Which One Of The Following Will Turn Red Litmus Blue
Mar 25, 2025
-
Standard Deviation And Coefficient Of Variation
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is Pseudo First Order Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
