What Is Group 17 On The Periodic Table Called
News Leon
Mar 22, 2025 · 6 min read
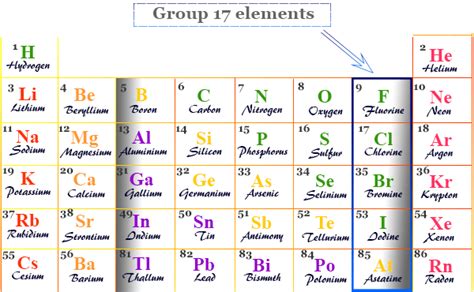
Table of Contents
What is Group 17 on the Periodic Table Called? A Deep Dive into the Halogens
Group 17 on the periodic table, also known as Group VIIA or VII, is famously called the halogens. This family of nonmetals possesses unique properties that make them crucial in various industrial processes and essential for life itself, albeit in carefully controlled amounts. Understanding the halogens requires exploring their physical and chemical characteristics, their reactivity, their applications, and their impact on the environment and human health.
Defining the Halogens: A Family Portrait
The term "halogen" originates from Greek words meaning "salt-former." This perfectly encapsulates their defining characteristic: their strong tendency to react with metals to form salts. The group includes five elements:
- Fluorine (F): The lightest and most reactive halogen.
- Chlorine (Cl): A greenish-yellow gas, widely used in disinfectants and water purification.
- Bromine (Br): The only non-metallic liquid element at room temperature, with a distinctive reddish-brown color.
- Iodine (I): A dark gray solid that sublimes (transitions directly from solid to gas) readily.
- Astatine (At): A radioactive, extremely rare element, with very limited practical applications.
While all are nonmetals, their physical states vary, reflecting the increasing atomic size and decreasing electronegativity as we move down the group. This variation in physical properties is directly tied to the strength of the intermolecular forces between atoms.
Electronic Configuration and Chemical Reactivity
The halogens share a common feature in their outermost electron shell: they all have seven valence electrons. This means they are only one electron short of achieving a stable octet configuration, the hallmark of noble gases. This electron deficiency drives their exceptionally high reactivity. They readily gain an electron to form a negatively charged ion, called a halide ion (F⁻, Cl⁻, Br⁻, I⁻, At⁻). This high electron affinity is the reason for their high electronegativity – their tendency to attract electrons in a chemical bond.
The reactivity of the halogens decreases down the group. Fluorine, being the smallest and most electronegative, is the most reactive. Its small size allows it to readily approach other atoms and attract electrons forcefully. As we move down the group, the atomic radius increases, and the outermost electrons become more shielded from the nucleus, reducing the electronegativity and reactivity.
Halogen Reactions: A Closer Look
The halogens participate in a wide range of chemical reactions. Their reactivity is particularly pronounced with metals and other nonmetals.
Reactions with Metals: Salt Formation
The quintessential reaction of halogens is their interaction with metals. They readily accept electrons from metals, forming ionic compounds known as halides. For example, the reaction of sodium (Na) with chlorine (Cl) produces sodium chloride (NaCl), common table salt.
2Na(s) + Cl₂(g) → 2NaCl(s)
This reaction, and similar ones involving other metals and halogens, highlights the halogens' role as excellent oxidizing agents—they readily accept electrons and are reduced in the process. The strength of the oxidizing power decreases down the group, with fluorine being the strongest oxidizing agent among the halogens.
Reactions with Nonmetals: Covalent Compound Formation
Halogens also react with other nonmetals to form covalent compounds, sharing electrons to achieve a stable octet. For example, chlorine reacts with hydrogen to form hydrogen chloride (HCl), a strong acid.
H₂(g) + Cl₂(g) → 2HCl(g)
These covalent compounds display diverse properties, depending on the nonmetal involved. For instance, carbon tetrachloride (CCl₄) is a nonpolar solvent, while phosphorus pentachloride (PCl₅) is a reactive compound used in organic synthesis.
Industrial Applications: The Versatility of Halogens
The unique chemical properties of the halogens translate into a wide range of industrial applications.
Chlorine: A Workhorse of Industry
Chlorine's versatility makes it a cornerstone in various industries:
- Water Treatment: Chlorine is a powerful disinfectant, crucial for purifying drinking water and wastewater, effectively eliminating harmful microorganisms.
- Bleaching: Chlorine-based bleaches are widely used in paper production and textile industries.
- PVC Production: Polyvinyl chloride (PVC), a widely used plastic, relies heavily on chlorine in its manufacturing process.
- Solvent Production: Chlorinated solvents, though increasingly regulated due to environmental concerns, find use in various industrial processes.
Fluorine: Specialized Applications
Fluorine's high reactivity limits its direct applications, but its compounds are incredibly important:
- Teflon (PTFE): Polytetrafluoroethylene, better known as Teflon, is a non-stick coating used in cookware and various industrial applications. Its exceptional thermal and chemical stability makes it invaluable.
- Refrigerants: Fluorocarbons were widely used as refrigerants, but their contribution to ozone depletion led to their phase-out under the Montreal Protocol. Hydrofluorocarbons (HFCs), less harmful alternatives, have partly replaced them, although they are still greenhouse gases.
- Dental Care: Fluoride, in low concentrations, is added to toothpaste and drinking water to strengthen tooth enamel and prevent cavities.
Bromine and Iodine: Specific Uses
Bromine and iodine also have specific niche applications:
- Bromine: Used in flame retardants, photographic chemicals, and certain pesticides.
- Iodine: Used as a disinfectant, in medical treatments (e.g., iodine tincture), and as a component in certain dyes.
It’s crucial to remember that while these applications are vital, the handling and disposal of halogen-containing compounds must be carefully managed to minimize environmental impact and health risks.
Environmental and Health Considerations: A Balanced Perspective
While the halogens offer numerous benefits, their reactivity and persistence in the environment raise crucial environmental and health concerns.
Ozone Depletion and Global Warming
Chlorofluorocarbons (CFCs) and other halogenated compounds were once widely used as refrigerants and propellants. However, their release into the atmosphere led to significant ozone depletion in the stratosphere, increasing the harmful UV radiation reaching Earth's surface. The Montreal Protocol, an international agreement, successfully phased out the production and consumption of ozone-depleting substances, leading to a slow but significant recovery of the ozone layer. While CFCs are being phased out, HFCs, although less damaging to the ozone layer, are potent greenhouse gases, contributing to global warming.
Toxicity and Environmental Persistence
Many halogenated compounds are toxic, posing risks to human health and the environment. Persistent organic pollutants (POPs), including some halogenated compounds, are resistant to degradation, persisting in the environment for extended periods, bioaccumulating in the food chain, and causing harm to wildlife and human health. Careful regulation and management of the production, use, and disposal of these compounds are critical.
Health Impacts
Direct exposure to certain halogens can have serious health consequences. For example, inhalation of chlorine gas can cause respiratory irritation and even death. Exposure to some halogenated compounds can lead to various health problems, including liver damage, kidney problems, and neurological effects. The specific health impacts vary significantly depending on the halogen, the chemical compound, the level of exposure, and the duration of exposure.
Conclusion: A Powerful Family with Complex Impacts
Group 17, the halogens, represent a vital yet complex family of elements. Their high reactivity makes them essential for countless applications, from purifying water to creating non-stick cookware. However, their reactivity also necessitates careful handling and management to mitigate potential risks to human health and the environment. Understanding their properties, their reactions, and their potential impacts is crucial for harnessing their benefits while minimizing the environmental and health challenges they present. The continued development of safer alternatives and responsible management practices will be essential for ensuring a sustainable future that benefits from the unique qualities of these remarkable elements. Further research into greener alternatives and efficient recycling processes remains essential in navigating the complex relationship between human needs and environmental sustainability in the context of halogen utilization. The balance between utilizing their powerful chemical properties and minimizing their potential harm is a persistent challenge that requires ongoing scientific and societal attention.
Latest Posts
Latest Posts
-
Why Does The Cyclops Invite Odysseus Back To The Island
Mar 23, 2025
-
Balance Equation C2h6 O2 Co2 H2o
Mar 23, 2025
-
What Is The Advantage Of Having Four Chambered Heart
Mar 23, 2025
-
Which Device Provides Electrical Energy To Run An Electric Circuit
Mar 23, 2025
-
Select The Statement That Best Describes Homeostasis
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is Group 17 On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
