What Is Density And Relative Density
News Leon
Mar 16, 2025 · 6 min read
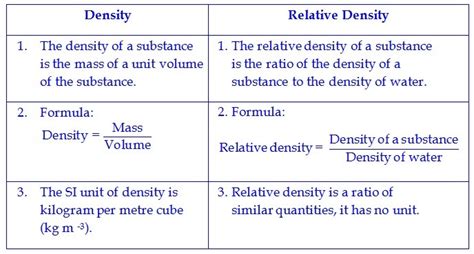
Table of Contents
What is Density and Relative Density? A Comprehensive Guide
Density and relative density are fundamental concepts in physics and materials science, crucial for understanding the properties of matter and its behavior in various contexts. While often used interchangeably in casual conversation, they represent distinct but related measurements. This comprehensive guide delves deep into the definitions, calculations, applications, and significance of both density and relative density. We'll explore their differences, practical examples, and how they play a critical role in numerous fields, from engineering and manufacturing to environmental science and medicine.
Understanding Density: Mass Packed into Space
Density, at its core, describes how compactly matter is packed into a given space. It quantifies the amount of mass present within a specific volume. More formally, density is defined as the mass per unit volume of a substance. The formula for calculating density is:
Density (ρ) = Mass (m) / Volume (V)
Where:
- ρ (rho): Represents density, usually measured in kilograms per cubic meter (kg/m³) in the SI system, or grams per cubic centimeter (g/cm³) which is equivalent. Other units, like pounds per cubic foot (lb/ft³), are also used depending on the context.
- m: Represents mass, typically measured in kilograms (kg) or grams (g). Mass is a measure of the amount of matter in an object.
- V: Represents volume, typically measured in cubic meters (m³) or cubic centimeters (cm³). Volume is the amount of three-dimensional space occupied by an object.
Factors Affecting Density:
Several factors influence the density of a substance:
- Temperature: Temperature changes affect the volume of a substance. Generally, increasing the temperature causes expansion, leading to a decrease in density. Conversely, decreasing the temperature causes contraction, increasing the density. This relationship is not always linear and can be influenced by the substance's phase (solid, liquid, or gas).
- Pressure: Pressure significantly affects the density of gases and, to a lesser extent, liquids. Increasing the pressure compresses the substance, reducing its volume and increasing its density.
- Composition: The chemical composition of a substance directly influences its density. Different atoms and molecules have different masses and packing arrangements, resulting in varying densities. For example, pure gold has a significantly higher density than pure aluminum.
Examples of Density in Everyday Life:
- Floating Objects: Objects float if their density is less than the density of the liquid they are placed in. A wooden block floats on water because its density is lower than that of water. Conversely, a metal coin sinks because its density is higher.
- Icebergs: Ice is less dense than liquid water, which is why icebergs float. This unusual property of water is crucial for aquatic life and the Earth's climate.
- Hot Air Balloons: Hot air is less dense than the surrounding cooler air, allowing hot air balloons to rise. This is a practical application of density differences.
Understanding Relative Density: Comparing Densities
Relative density, also known as specific gravity, is a dimensionless ratio that compares the density of a substance to the density of a reference substance. The reference substance is typically water at 4°C (39.2°F), at which its density is approximately 1000 kg/m³ or 1 g/cm³.
Relative Density (RD) = Density of Substance / Density of Reference Substance
Since the relative density is a ratio of two densities, it's a dimensionless quantity. This means it doesn't have units. A relative density of 2 indicates that the substance is twice as dense as the reference substance (water). A relative density less than 1 indicates that the substance is less dense than the reference substance.
Importance of Relative Density:
Relative density is a very useful property because:
- Easy Comparison: It allows for easy comparison of the densities of different substances without needing to know their absolute densities in various units. It simplifies density comparisons across different measurement systems.
- Practical Applications: It's particularly useful in gemology, where the relative density helps identify different gemstones. It is also used extensively in the petroleum industry to determine the density of crude oil and its various components.
- Simplified Calculations: In many calculations involving buoyancy and fluid mechanics, using relative density simplifies the equations.
Examples of Relative Density Applications:
- Gem Identification: Gemologists use relative density measurements to help distinguish between different gemstones, as each gemstone has a unique relative density. This is a crucial tool for authenticating precious stones.
- Hydrometer: A hydrometer is a simple instrument used to measure the relative density of liquids. This is commonly used in brewing, winemaking, and other industries where the density of the liquid is crucial for quality control.
- Soil Analysis: Relative density measurements are employed in soil analysis to determine the compaction and porosity of soils, crucial for construction and agricultural purposes.
Density vs. Relative Density: Key Differences
While both density and relative density relate to the "heaviness" of a substance, there are crucial differences:
| Feature | Density | Relative Density |
|---|---|---|
| Definition | Mass per unit volume | Ratio of a substance's density to a reference density |
| Units | kg/m³, g/cm³, lb/ft³ etc. | Dimensionless |
| Value | Can range from very small to very large | Typically ranges from 0 to values greater than 1 |
| Reference | No reference substance required | Uses a reference substance (usually water at 4°C) |
| Applications | Wide range, including material science, fluid mechanics, etc. | Often used for comparison and identification |
Advanced Concepts and Applications
The concepts of density and relative density extend beyond basic calculations and find applications in many advanced fields:
1. Archimedes' Principle and Buoyancy:
Archimedes' principle states that an object submerged in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced by the object. This buoyant force depends on the density of the fluid and the volume of the object submerged. Objects with a density less than the fluid float, while those with a greater density sink. Relative density plays a crucial role in understanding this principle.
2. Fluid Mechanics and Hydrostatics:
Density is a critical parameter in fluid mechanics, governing the behavior of fluids under various conditions. Equations describing pressure, flow, and buoyancy heavily rely on the density of the fluids involved. Relative density facilitates comparison of fluid behavior under different conditions.
3. Materials Science and Engineering:
Density is a key material property considered in engineering design. The choice of material often depends on its density in applications where weight is a critical factor, such as aerospace engineering and automotive design. Knowing the relative density helps compare different materials for specific applications.
4. Environmental Science:
Density differences are exploited in various environmental applications, such as water treatment (separation of solids from liquids based on density) and pollution monitoring (measuring the density of pollutants in air or water).
5. Medical Applications:
Density measurements are used in medical imaging techniques like bone densitometry to assess bone health. Specific gravity of urine can indicate kidney function, demonstrating the practical use of relative density in medical diagnosis.
Conclusion: Practical Importance and Ongoing Relevance
Density and relative density are fundamental concepts with wide-ranging applications across numerous scientific and engineering disciplines. Understanding these concepts is crucial for comprehending the properties of matter, fluid behavior, and the interaction between objects and fluids. From the design of aircraft to the identification of gemstones and the monitoring of environmental pollution, these seemingly simple measurements have profound practical implications and continue to be crucial in advancing scientific knowledge and technological innovation. Their dimensionless nature in the case of relative density simplifies comparisons and calculations, highlighting their enduring value in diverse fields. As our understanding of materials and fluid dynamics continues to evolve, the importance of density and relative density will only increase.
Latest Posts
Latest Posts
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is Density And Relative Density . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
