What Is Conjugate Base Of Hso4-
News Leon
Mar 19, 2025 · 5 min read
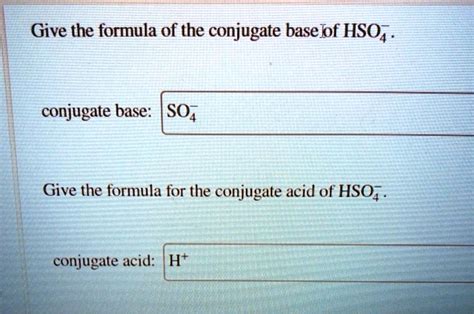
Table of Contents
What is the Conjugate Base of HSO₄⁻? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article will delve deep into the concept, specifically focusing on the conjugate base of the bisulfate ion, HSO₄⁻. We'll explore its properties, reactions, and significance in various chemical contexts. By the end, you'll have a comprehensive understanding of this important chemical species and its role in equilibrium reactions.
Understanding Conjugate Acid-Base Pairs
Before we pinpoint the conjugate base of HSO₄⁻, let's establish a solid foundation. According to Brønsted-Lowry acid-base theory, an acid is a proton (H⁺) donor, while a base is a proton acceptor. A crucial aspect of this theory is the concept of conjugate acid-base pairs.
When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are always related by the difference of a single proton (H⁺).
Think of it like this: An acid loses a proton to become its conjugate base, and a base gains a proton to become its conjugate acid. They are essentially two species that differ by only one proton.
Identifying the Conjugate Base of HSO₄⁻
Now, let's focus on the bisulfate ion, HSO₄⁻. This ion acts as a weak acid in aqueous solutions. This means it only partially dissociates, donating a proton to water molecules to a limited extent. The dissociation reaction is represented as follows:
HSO₄⁻(aq) + H₂O(l) ⇌ SO₄²⁻(aq) + H₃O⁺(aq)
In this reaction, HSO₄⁻ donates a proton (H⁺) to a water molecule (H₂O), forming the hydronium ion (H₃O⁺). The species remaining after HSO₄⁻ loses its proton is SO₄²⁻, the sulfate ion. Therefore, the conjugate base of HSO₄⁻ is SO₄²⁻.
Properties of the Sulfate Ion (SO₄²⁻)
The sulfate ion, SO₄²⁻, is a polyatomic anion composed of one sulfur atom and four oxygen atoms. It carries a -2 charge, indicating it has gained two electrons. Understanding its properties is crucial to understanding its behavior as the conjugate base of HSO₄⁻.
-
Solubility: Sulfate salts are generally soluble in water, except for those of barium, strontium, lead, and calcium. This solubility is significant in various applications, from industrial processes to biological systems.
-
Reactivity: As a conjugate base, SO₄²⁻ can accept a proton. However, it is a relatively weak base, meaning its tendency to accept a proton is limited. It's much less likely to abstract a proton than, say, the hydroxide ion (OH⁻).
-
Structure: The sulfate ion has a tetrahedral structure, with the sulfur atom at the center and four oxygen atoms surrounding it. This structure contributes to its stability and its relatively weak basicity. The negative charges are delocalized across the four oxygen atoms, enhancing stability.
Reactions Involving HSO₄⁻ and SO₄²⁻
The equilibrium between HSO₄⁻ and SO₄²⁻ is crucial in many chemical reactions. The position of this equilibrium depends on the pH of the solution.
-
In acidic solutions: The equilibrium shifts to the left, favoring the formation of HSO₄⁻. The high concentration of H⁺ ions suppresses the dissociation of HSO₄⁻.
-
In basic solutions: The equilibrium shifts to the right, favoring the formation of SO₄²⁻. The low concentration of H⁺ ions promotes the dissociation of HSO₄⁻.
This equilibrium plays a significant role in buffer solutions. A buffer solution is a solution that resists changes in pH upon the addition of small amounts of acid or base. A mixture of HSO₄⁻ and SO₄²⁻ can form a buffer solution, effectively controlling the pH within a specific range. This characteristic is exploited in many applications, including biological systems and chemical industries.
Significance of HSO₄⁻ and SO₄²⁻ in Various Contexts
The bisulfate ion (HSO₄⁻) and its conjugate base, the sulfate ion (SO₄²⁻), play important roles in various fields:
-
Industrial Applications: Sulfuric acid (H₂SO₄), a strong acid, is a crucial industrial chemical. HSO₄⁻ is an intermediate species in many sulfuric acid reactions. Sulfates are used extensively in various industries, including fertilizers, detergents, and pharmaceuticals.
-
Environmental Science: Sulfate ions are present in rainwater, contributing to acid rain. Understanding the acid-base chemistry of HSO₄⁻ and SO₄²⁻ is crucial for mitigating the environmental impacts of acid rain.
-
Biological Systems: Sulfate ions are involved in various biological processes. They're essential components of some molecules and play a role in enzyme activity and metabolic pathways.
-
Analytical Chemistry: Titration involving HSO₄⁻ and its salts is often used in analytical chemistry to determine the concentration of various substances.
Understanding the pKa Value
The pKa value provides a quantitative measure of the strength of an acid. It represents the negative logarithm of the acid dissociation constant (Ka). A lower pKa value indicates a stronger acid.
The pKa of HSO₄⁻ is approximately 1.99. This relatively low pKa value confirms its status as a weak acid. The higher the pKa value of the acid, the weaker the acid and the stronger its conjugate base.
Distinguishing between HSO₄⁻ and SO₄²⁻
It's vital to differentiate between HSO₄⁻ and SO₄²⁻. While they are chemically related, they possess distinct properties and behave differently in various reactions:
-
Charge: HSO₄⁻ carries a -1 charge, while SO₄²⁻ carries a -2 charge. This difference significantly impacts their reactivity and solubility.
-
Acidity/Basicity: HSO₄⁻ is a weak acid, while SO₄²⁻ is a weak base. Their different tendencies to donate or accept protons lead to different reaction outcomes.
-
Reactions: HSO₄⁻ participates in reactions as a proton donor, while SO₄²⁻ participates as a proton acceptor.
Conclusion: Mastering the Conjugate Base of HSO₄⁻
The conjugate base of HSO₄⁻ is SO₄²⁻, the sulfate ion. Understanding the relationship between these two species is fundamental to comprehending acid-base chemistry. Their properties, reactions, and significance in various contexts highlight their importance across many scientific disciplines. By grasping the concepts discussed in this article, you can confidently address complex chemical scenarios involving the bisulfate and sulfate ions and appreciate the broader implications of conjugate acid-base pairs in chemical equilibrium. This knowledge extends beyond simple textbook examples to real-world applications in diverse fields, emphasizing the practical relevance of this fundamental chemical concept. The subtle differences and crucial roles of these ions underscore the importance of detailed study in chemical understanding.
Latest Posts
Latest Posts
-
The Urinary Bladder And Ureters Are Lined By
Mar 19, 2025
-
How Many Light Years Is The Moon From The Earth
Mar 19, 2025
-
Mending Wall Line By Line Analysis
Mar 19, 2025
-
What Is The Specific Heat Capacity Of Aluminum
Mar 19, 2025
-
What Is The Reciprocal Of 14
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is Conjugate Base Of Hso4- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
