What Is An Independent Variable In Chemistry
News Leon
Mar 19, 2025 · 5 min read
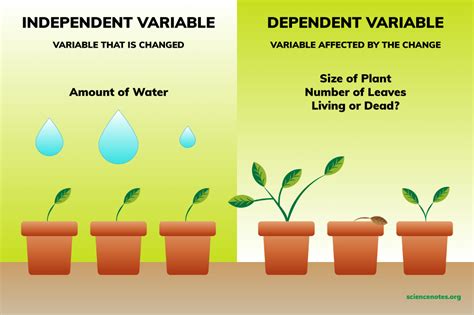
Table of Contents
What is an Independent Variable in Chemistry? A Comprehensive Guide
Understanding variables is fundamental to conducting and interpreting scientific experiments, especially in chemistry. This article delves deep into the concept of the independent variable in chemistry, explaining its definition, role in experiments, how to identify it, examples, and its relationship with other variables like dependent and controlled variables. We'll also explore common misconceptions and provide practical tips for effectively using independent variables in your chemical investigations.
Defining the Independent Variable
In the context of a chemical experiment, the independent variable is the factor that is deliberately manipulated or changed by the experimenter. It's the variable that you, the researcher, have control over and actively alter to observe its effect on other variables. Think of it as the cause in a cause-and-effect relationship. It's the variable you are testing to see how it impacts the outcome of your experiment. It is also often referred to as the manipulated variable or the predictor variable.
Key characteristics of an independent variable:
- Controlled by the researcher: The researcher directly changes or selects the values of the independent variable.
- Predictive influence: It is expected to have an effect on the dependent variable.
- Multiple levels or values: To observe a relationship, the independent variable is usually tested at multiple levels or values. For example, you might test the reaction rate at different temperatures (e.g., 25°C, 50°C, 75°C).
The Role of the Independent Variable in Experiments
The independent variable plays a crucial role in establishing cause-and-effect relationships in chemical experiments. By systematically changing its value, you can observe the corresponding changes in the dependent variable, enabling you to determine whether a relationship exists between them. This methodical approach is the cornerstone of the scientific method.
A well-designed experiment needs a clearly defined independent variable. Without a clearly identified independent variable, it is impossible to determine what is causing any observed changes. This clarity is vital for ensuring the validity and reproducibility of your experimental findings.
Identifying the Independent Variable in Chemical Experiments
Identifying the independent variable often comes down to asking yourself: "What am I changing or manipulating in this experiment?" The answer to this question is usually your independent variable.
Consider some examples:
- Experiment: Investigating the effect of temperature on the rate of a chemical reaction.
- Independent Variable: Temperature. The researcher controls the temperature at which the reaction occurs.
- Experiment: Determining the effect of different catalysts on the yield of a product.
- Independent Variable: Type of catalyst. The researcher chooses which catalysts to use.
- Experiment: Examining the solubility of a solute at different concentrations.
- Independent Variable: Concentration of the solute. The researcher prepares solutions with varying solute concentrations.
- Experiment: Measuring the pH of a solution after adding varying amounts of acid.
- Independent Variable: Amount of acid added. The researcher controls the volume of acid introduced to the solution.
Independent Variable vs. Dependent Variable vs. Controlled Variable
It’s vital to differentiate the independent variable from other variables in your experiment. Confusing these roles can lead to misinterpretations of your results.
-
Dependent Variable: This is the variable that is measured or observed. It's the variable that depends on the changes made to the independent variable. It's the effect in the cause-and-effect relationship. In our examples above, the dependent variables would be reaction rate, product yield, solubility, and pH respectively.
-
Controlled Variables: These are the factors that are kept constant throughout the experiment to prevent them from influencing the dependent variable. Controlling these variables ensures that any observed changes in the dependent variable are directly attributable to the independent variable. In the temperature experiment, controlled variables could include the amount of reactants, pressure, and the type of reaction vessel.
Common Misconceptions about Independent Variables
Several misconceptions surround independent variables, particularly for those new to scientific experimentation:
-
Confusing independent and dependent variables: This is a very common mistake. Remember, the independent variable is what you change, and the dependent variable is what you measure.
-
Having too many independent variables: While exploring multiple factors is important, having too many independent variables in a single experiment makes it difficult to isolate the effect of each variable. It's better to focus on one or two independent variables at a time.
-
Not controlling other variables: Failing to control other variables can lead to inaccurate conclusions. Extraneous variables can confound the results, making it impossible to determine the true effect of the independent variable.
Advanced Concepts and Applications
The understanding of independent variables extends beyond simple experiments. In more advanced chemical investigations, you might encounter:
-
Quantitative vs. Qualitative Independent Variables: Quantitative variables are measured numerically (e.g., temperature, concentration). Qualitative variables are categorical (e.g., type of catalyst, presence/absence of a substance).
-
Multiple Independent Variables: Some experiments involve multiple independent variables to explore complex interactions. Statistical techniques like ANOVA (Analysis of Variance) are often used to analyze data from such experiments.
-
Factorial Designs: These experimental designs systematically vary multiple independent variables to examine their individual and combined effects on the dependent variable.
Practical Tips for Using Independent Variables Effectively
- Clearly define your independent variable: Be precise in stating what you're manipulating.
- Choose appropriate levels or values: Select a range of values that will effectively demonstrate the relationship between the independent and dependent variables.
- Control other variables: Minimize the influence of extraneous variables through careful experimental design and control measures.
- Replicate your experiments: Repeating the experiment with the same independent variable values will increase the reliability and validity of your findings.
- Analyze your data appropriately: Use statistical methods to analyze the relationship between the independent and dependent variables.
Conclusion
Understanding the independent variable is crucial for conducting meaningful chemical experiments. By carefully selecting and manipulating the independent variable while controlling other factors, you can reliably investigate cause-and-effect relationships and gain valuable insights into chemical phenomena. Remember to clearly define your variables, control extraneous factors, and analyze your data rigorously to draw accurate and meaningful conclusions. The careful consideration of independent variables is a key component of scientific rigor and successful chemical experimentation. Mastering this concept opens the door to a deeper understanding of the chemical world and the power of scientific inquiry.
Latest Posts
Latest Posts
-
Mending Wall Line By Line Analysis
Mar 19, 2025
-
What Is The Specific Heat Capacity Of Aluminum
Mar 19, 2025
-
What Is The Reciprocal Of 14
Mar 19, 2025
-
What Are The Raw Materials Required For Photosynthesis
Mar 19, 2025
-
Which Of The Following Is Not An Organic Substance
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is An Independent Variable In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
