What Is An Electron Sea Model
News Leon
Mar 27, 2025 · 6 min read
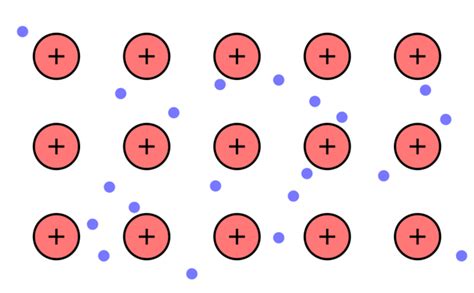
Table of Contents
What is the Electron Sea Model? A Deep Dive into Metallic Bonding
The electron sea model, also known as the free electron model, is a simple yet powerful way to understand the behavior of metals and their unique properties. Unlike ionic or covalent bonding where electrons are localized to specific atoms or shared between them, the electron sea model proposes a radically different picture: a lattice of positive ions submerged in a "sea" of delocalized electrons. This model elegantly explains many characteristic properties of metals, such as their excellent conductivity, malleability, ductility, and metallic luster. This article will delve deep into the electron sea model, exploring its postulates, limitations, and applications.
Understanding the Postulates of the Electron Sea Model
At the heart of the electron sea model lies a set of fundamental assumptions:
-
Positive Ion Core: Metals are depicted as a regular lattice structure of positively charged ions (cations). These ions are formed when metal atoms lose their valence electrons. The positive charge of these ions is crucial for the overall stability of the structure. The specific arrangement of these ions depends on the metal's crystal structure (e.g., body-centered cubic, face-centered cubic, hexagonal close-packed).
-
Delocalized Electrons: The valence electrons, those in the outermost shell of the metal atom, are not associated with any particular ion. Instead, they are free to move throughout the entire lattice, forming a "sea" of electrons. This delocalization is the key feature differentiating metallic bonding from other types of chemical bonding.
-
Electrostatic Attraction: The positively charged ion cores are held together by the electrostatic attraction between the positive ions and the negatively charged electron sea. This attraction is non-directional, meaning it isn't confined to specific bonds between individual atoms, unlike covalent bonds. This non-directional nature is crucial for explaining the malleability and ductility of metals.
-
Electron Mobility: The electrons in the sea are highly mobile and can easily move under the influence of an electric field. This mobility is responsible for the excellent electrical and thermal conductivity of metals. The electrons can readily transport charge and energy throughout the material.
Explaining Metallic Properties Using the Electron Sea Model
The electron sea model effectively explains several key properties of metals:
1. Electrical Conductivity:
The high electrical conductivity of metals is a direct consequence of the mobile electron sea. When an electric field is applied across a metal, the delocalized electrons readily move in the direction of the field, constituting an electric current. This free flow of electrons results in metals being excellent conductors of electricity, a property crucial in countless applications from power transmission to electronics.
2. Thermal Conductivity:
Similarly, the electron sea accounts for the high thermal conductivity of metals. The mobile electrons can easily transfer thermal energy throughout the lattice, allowing heat to be efficiently conducted through the material. This explains why metals feel cold to the touch – they readily absorb heat from their surroundings.
3. Malleability and Ductility:
Metals are known for their malleability (the ability to be hammered into sheets) and ductility (the ability to be drawn into wires). The electron sea model explains this by the non-directional nature of the metallic bond. When a metal is deformed, the positively charged ions can slide past each other without disrupting the overall structure. The electron sea acts as a "glue" that maintains the cohesion of the metal despite the rearrangement of the ions. This contrasts sharply with ionic and covalent materials where specific bond directions lead to brittleness upon deformation.
4. Metallic Luster:
The shiny appearance or metallic luster of metals is also attributed to the electron sea. The delocalized electrons can absorb and re-emit light across a wide range of frequencies, leading to the characteristic reflection of light we associate with metals. The specific color of the metallic luster depends on the electron configuration of the metal and how it interacts with light.
5. High Melting and Boiling Points:
Many metals exhibit high melting and boiling points. This is because the electrostatic attraction between the positive ions and the electron sea is strong, requiring a significant amount of energy to overcome these forces and break down the metallic lattice. The strength of this interaction is determined by the number of valence electrons and the charge density of the ions.
Limitations of the Electron Sea Model
Despite its successes, the electron sea model has its limitations:
-
Oversimplification: The model treats electrons as completely free and delocalized, neglecting the interaction between electrons and the ion cores. In reality, there is some interaction, and the electrons are not entirely free.
-
Band Theory: A more sophisticated model, band theory, provides a more accurate description of metallic bonding by considering the energy levels of electrons within the metal. The electron sea model doesn't account for the energy bands and their structure.
-
Magnetic Properties: The electron sea model struggles to fully explain the magnetic properties of some metals, such as ferromagnetism (permanent magnetism). More advanced models, incorporating electron spin and exchange interactions, are needed to understand these phenomena.
-
Alloy Behavior: While it can qualitatively explain some aspects of alloy behavior, it lacks the precision to predict the properties of alloys accurately. The interactions between different types of metal atoms in an alloy are not straightforwardly modeled by the simple electron sea picture.
Advancements and Related Models
The electron sea model has paved the way for more sophisticated models of metallic bonding, such as:
-
Band Theory: This quantum mechanical model considers the energy levels of electrons in a metal, describing them as forming continuous bands of allowed energies. This explains features like electrical conductivity and the difference between conductors, semiconductors, and insulators more accurately than the simple electron sea model.
-
Density Functional Theory (DFT): DFT is a powerful computational method used to model the electronic structure of materials, including metals. It provides a more accurate and detailed description of electron distribution and bonding than simpler models.
Conclusion: The Electron Sea Model – A Foundation for Understanding Metals
The electron sea model, despite its limitations, remains a valuable and intuitive tool for understanding the fundamental properties of metals. It provides a simple yet effective explanation for their excellent conductivity, malleability, ductility, and metallic luster. While more sophisticated models like band theory and DFT offer a more complete description, the electron sea model provides a crucial foundation for grasping the essential characteristics of metallic bonding and the behavior of metals in various applications. Its simplicity makes it an excellent starting point for students learning about chemical bonding and the properties of materials, highlighting the power of simplified models in elucidating complex phenomena. It encourages further exploration into the more nuanced and complex aspects of metallic bonding through its introductory explanation. The model's impact on our understanding of materials science is undeniable, contributing to advancements in various fields relying on the unique properties of metals.
Latest Posts
Latest Posts
-
An Object Of Mass M Is Thrown Vertically Upward
Mar 30, 2025
-
What Is Found In Both Dna And Rna
Mar 30, 2025
-
How Many Eyelids Does A Frog Have
Mar 30, 2025
-
Do Birds Have Four Chambered Heart
Mar 30, 2025
-
The Demand Curve For A Monopolist Is
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is An Electron Sea Model . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
