What Is An Aliquot In Chemistry
News Leon
Mar 24, 2025 · 7 min read
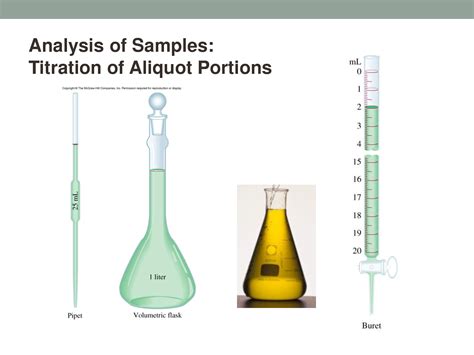
Table of Contents
What is an Aliquot in Chemistry? A Comprehensive Guide
Understanding aliquots is crucial for accurate and reliable results in various chemical analyses. This comprehensive guide will delve into the definition, importance, and practical applications of aliquots in chemistry, equipping you with the knowledge to confidently utilize this technique in your work.
Defining an Aliquot in Chemistry
In chemistry, an aliquot is a precisely measured portion of a larger sample. It's a known fraction of the original solution, carefully separated to perform analysis or further dilutions. The process of taking an aliquot ensures that the smaller sample accurately represents the composition of the original, larger sample. This is critical for maintaining accuracy and reproducibility in experiments. Think of it as taking a representative sample from a larger batch to save time and resources, while still obtaining reliable data.
The term "aliquot" implies precision and accuracy. Unlike taking a random sample, an aliquot is measured using calibrated instruments, ensuring the volume is known with a high degree of certainty. This meticulous measurement is the cornerstone of quantitative analysis and ensures the reliability of experimental findings.
Why Use Aliquots?
The use of aliquots is widespread in chemistry for several compelling reasons:
-
Sample size reduction: Dealing with large sample volumes can be impractical, time-consuming, and resource-intensive. Aliquots allow researchers to work with smaller, manageable portions while maintaining data integrity.
-
Multiple analyses: A single, carefully prepared solution can be used to create multiple aliquots. This enables researchers to perform various analyses on the same sample, saving time and ensuring consistency.
-
Serial dilutions: Aliquots are essential for creating serial dilutions – a series of solutions with progressively decreasing concentrations. This technique is widely used in various analytical methods to achieve the desired concentration range for analysis.
-
Improved accuracy and precision: By using calibrated instruments to measure the aliquot, the error associated with sample preparation is significantly reduced, leading to more accurate and precise results.
-
Avoiding contamination: Working with smaller aliquots minimizes the risk of contamination during the analysis process.
The Process of Taking an Aliquot
The procedure for taking an aliquot involves several key steps, requiring careful attention to detail:
1. Preparation of the Stock Solution
The process begins with the preparation of a stock solution, which is a solution of known concentration. This solution is usually prepared by dissolving a precisely weighed amount of solute in a specific volume of solvent. The concentration of the stock solution must be accurately determined to ensure the reliability of subsequent aliquots.
2. Thorough Mixing
Before taking an aliquot, the stock solution must be thoroughly mixed. This step is crucial to ensure homogeneity and prevent inaccuracies due to uneven distribution of the solute. Gentle swirling or inversion is usually sufficient. Vigorous shaking can introduce errors.
3. Using Appropriate Volumetric Glassware
The choice of glassware plays a significant role in the accuracy of the aliquot. Commonly used equipment includes:
-
Volumetric pipettes: These are designed to deliver a precise volume of liquid. They are ideal for taking aliquots when high accuracy is required.
-
Volumetric flasks: Used for preparing solutions of known concentration. The aliquot is often taken from a solution prepared in a volumetric flask.
-
Burettes: These allow for the dispensing of variable volumes of liquid and are suitable for taking aliquots when precise volume control is needed.
-
Graduated cylinders: While less precise than pipettes or burettes, they can be used for taking aliquots when high accuracy is not critical.
Selecting the appropriate glassware depends on the required level of accuracy and the volume of the aliquot. For instance, volumetric pipettes offer the highest precision, while graduated cylinders are acceptable for less demanding applications.
4. Transferring the Aliquot
Once the appropriate glassware is selected, the aliquot is carefully transferred. This process should minimize spillage and ensure that the entire aliquot is transferred. For pipettes, this involves proper pipetting technique to avoid introducing air bubbles or leaving liquid behind.
5. Dilution (if necessary)
After transferring the aliquot, it may be necessary to dilute it to achieve the desired concentration for analysis. This dilution must be performed carefully, using calibrated volumetric glassware to ensure accuracy.
Applications of Aliquots in Chemistry
The applications of aliquots are extensive and span various fields of chemistry:
1. Analytical Chemistry
Aliquots are indispensable in analytical chemistry for various techniques, including:
-
Titration: Aliquots are used to prepare samples for titration, allowing for multiple titrations on the same sample for improved accuracy.
-
Spectrophotometry: Aliquots are used to prepare samples for spectrophotometric analysis. The diluted aliquot ensures the absorbance falls within the optimal range for measurement.
-
Chromatography: Aliquots of samples are injected into the chromatographic system for separation and analysis of various components.
-
Atomic Absorption Spectroscopy (AAS): Aliquots of diluted samples are analyzed to determine the concentration of specific elements.
-
Inductively Coupled Plasma Mass Spectrometry (ICP-MS): Aliquots are used to prepare samples for analysis by ICP-MS, a highly sensitive technique for determining the concentration of trace elements.
2. Biochemistry and Molecular Biology
In biochemistry and molecular biology, aliquots are essential for:
-
Enzyme assays: Aliquots of enzyme solutions and substrates are used to measure enzyme activity.
-
Protein quantification: Aliquots of protein solutions are used for determining protein concentration.
-
DNA and RNA analysis: Aliquots are used for preparing samples for various DNA and RNA analysis techniques, such as PCR and electrophoresis.
3. Environmental Chemistry
Aliquots are frequently used in environmental chemistry to analyze:
-
Water samples: Aliquots of water samples are analyzed to determine the concentration of pollutants and other constituents.
-
Soil samples: Aliquots of soil samples are analyzed for the presence of heavy metals and other contaminants.
-
Air samples: Aliquots of collected air samples are analyzed to determine the concentration of various gases and pollutants.
4. Pharmaceutical Chemistry
In pharmaceutical chemistry, aliquots are vital for:
-
Drug formulation: Aliquots are used to prepare samples for analysis to ensure drug quality and purity.
-
Drug stability testing: Aliquots are used to test drug stability over time.
-
Bioavailability studies: Aliquots are used to study drug absorption and distribution in the body.
Importance of Accuracy and Precision in Aliquot Handling
The accuracy and precision of aliquot handling are paramount for the reliability of the results obtained. Any errors introduced during the process can significantly affect the final results. Therefore, meticulous attention to detail is crucial, including:
-
Proper calibration of glassware: Regular calibration of volumetric glassware is essential to ensure accuracy.
-
Correct pipetting technique: Using proper pipetting technique is crucial to avoid introducing errors.
-
Avoiding contamination: Contamination can significantly affect the results, so proper cleanliness and handling procedures must be followed.
-
Using appropriate techniques for mixing solutions: Vigorous shaking should be avoided to prevent the formation of air bubbles or foaming, which can affect the accuracy of the volume measurement.
Troubleshooting Common Issues with Aliquot Preparation
While the process of preparing and handling aliquots is generally straightforward, some common issues may arise:
-
Inaccurate measurements: This can be due to incorrect calibration of glassware, improper pipetting technique, or errors in calculations. Regular calibration and careful technique are vital to avoid this.
-
Contamination: This can lead to inaccurate results. Maintaining a clean working environment and using appropriate techniques for handling samples are crucial to prevent contamination.
-
Inhomogeneous solutions: If the stock solution is not thoroughly mixed before taking an aliquot, the resulting aliquot may not accurately represent the composition of the whole sample. Thorough mixing is essential to ensure homogeneity.
-
Loss of sample during transfer: Care should be taken during the transfer of the aliquot to prevent spillage or loss of sample. Using appropriate techniques, such as using a pipette filler, minimizes the risk of error.
By addressing these potential issues proactively, researchers can ensure the accuracy and reliability of their results.
Conclusion
In conclusion, aliquots are essential tools in various chemical analyses, providing a practical means of working with manageable sample portions while maintaining the integrity of the original sample. Understanding the principles and techniques of aliquot preparation and handling is vital for accurate and reproducible results in any chemical experiment. Careful attention to detail, proper calibration of glassware, and the use of appropriate techniques are crucial for minimizing errors and ensuring the reliability of chemical analyses. The widespread use of aliquots across diverse fields underscores their importance in scientific research and technological advancements.
Latest Posts
Latest Posts
-
Dna Is Composed Of Repeating Structural Units Called
Mar 25, 2025
-
Which Substance Loses Electrons In A Chemical Reaction
Mar 25, 2025
-
1 Mg Is How Many Units
Mar 25, 2025
-
Uaa Uga And Uag Are All Codons
Mar 25, 2025
-
Sodium Is A Solid Liquid Or Gas
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is An Aliquot In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
