What Is An Abbreviated Electron Configuration
News Leon
Mar 21, 2025 · 6 min read
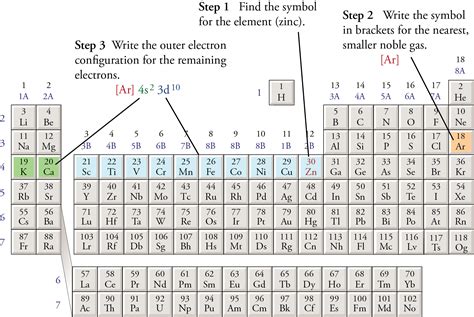
Table of Contents
What is an Abbreviated Electron Configuration? A Comprehensive Guide
Understanding electron configuration is fundamental to grasping the behavior of atoms and their interactions. While the full electron configuration provides a complete picture of electron arrangement, the abbreviated electron configuration offers a more concise and efficient way to represent the same information, particularly for larger atoms. This guide delves into the intricacies of abbreviated electron configurations, explaining what they are, how to write them, and their significance in chemistry.
What is Electron Configuration?
Before diving into abbreviated configurations, let's establish a firm understanding of the standard electron configuration. The electron configuration of an atom describes the arrangement of electrons in its various energy levels (shells) and sublevels (subshells). These arrangements are governed by specific rules:
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level and progressing to higher levels.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins.
- Hund's Rule: Electrons will individually occupy each orbital within a subshell before doubling up in any one orbital.
These rules dictate the order in which electrons fill the orbitals: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on. Each orbital is represented by a notation indicating the principal quantum number (energy level, n), the type of subshell (s, p, d, or f), and the number of electrons in that subshell (indicated as a superscript).
For example, the full electron configuration of oxygen (atomic number 8) is 1s²2s²2p⁴. This tells us that oxygen has two electrons in the 1s orbital, two in the 2s orbital, and four in the 2p orbitals.
The Need for Abbreviated Electron Configurations
Writing out the full electron configuration for elements with high atomic numbers can become lengthy and cumbersome. This is where the abbreviated electron configuration proves incredibly useful. It simplifies the notation by utilizing the noble gas configuration as a shorthand representation.
Noble gases are the elements in Group 18 of the periodic table. They are characterized by their extremely stable electron configurations, with completely filled valence shells. This stability is why they are relatively unreactive. The abbreviated electron configuration leverages this stability to shorten the notation.
How to Write an Abbreviated Electron Configuration
The process involves finding the noble gas that precedes the element in question on the periodic table. The electron configuration of this noble gas represents the core electrons of the element. The remaining electrons, which are located in the valence shell, are then written explicitly.
Let's illustrate this with examples:
Example 1: Sodium (Na, atomic number 11)
The full electron configuration of sodium is 1s²2s²2p⁶3s¹. The noble gas preceding sodium is neon (Ne), which has the electron configuration 1s²2s²2p⁶. Therefore, the abbreviated electron configuration of sodium is [Ne]3s¹. The [Ne] represents the core electrons, while 3s¹ represents the valence electron.
Example 2: Iron (Fe, atomic number 26)
The full electron configuration of iron is 1s²2s²2p⁶3s²3p⁶4s²3d⁶. The noble gas preceding iron is argon (Ar), with the electron configuration 1s²2s²2p⁶3s²3p⁶. Thus, the abbreviated electron configuration of iron is [Ar]4s²3d⁶.
Example 3: Iodine (I, atomic number 53)
Iodine's full electron configuration is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁵. The noble gas preceding iodine is krypton (Kr), with the electron configuration 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶. Therefore, the abbreviated electron configuration of iodine is [Kr]5s²4d¹⁰5p⁵.
Important Note: The order of filling orbitals sometimes deviates slightly from the simple Aufbau principle, especially for transition metals and inner transition metals. However, the abbreviated configuration still follows the same principle of using the preceding noble gas as the core.
Significance of Abbreviated Electron Configurations
The use of abbreviated electron configurations offers several advantages:
- Conciseness: It provides a more compact and efficient way to represent the electron configuration, especially for elements with many electrons.
- Clarity: It emphasizes the valence electrons, which are crucial in determining an element's chemical properties and reactivity. Valence electrons are those in the outermost shell and are primarily involved in bonding.
- Predictability of Properties: The similarity in valence electron configurations between elements in the same group explains the periodic trends observed in their properties. Elements with similar valence electron configurations will exhibit similar chemical behaviors.
- Ease of Comparison: Comparing abbreviated configurations readily highlights similarities and differences between elements, facilitating understanding of chemical bonding and reactivity.
Exceptions to the Standard Filling Order
While the Aufbau principle generally predicts the electron configuration, there are exceptions, particularly among transition metals and lanthanides/actinides. These exceptions arise due to the energy differences between subshells being relatively small, leading to slight variations in electron filling.
For instance, chromium (Cr) and copper (Cu) exhibit unexpected electron configurations:
- Chromium (Cr, atomic number 24): The predicted configuration is [Ar]4s²3d⁴, but the actual configuration is [Ar]4s¹3d⁵. This deviation results from the extra stability gained by having a half-filled d subshell (five electrons).
- Copper (Cu, atomic number 29): The predicted configuration is [Ar]4s²3d⁹, but the actual configuration is [Ar]4s¹3d¹⁰. This arises from the increased stability associated with a completely filled d subshell (ten electrons).
These exceptions highlight the limitations of the simple Aufbau principle but don't invalidate the concept of abbreviated electron configurations. The abbreviated configuration still provides a valuable and efficient representation, even with these deviations.
Applications of Abbreviated Electron Configurations
Abbreviated electron configurations are not merely a shorthand notation; they are essential tools in various chemical concepts:
- Predicting Chemical Bonding: The valence electrons, clearly shown in the abbreviated configuration, determine how an atom will bond with other atoms. For example, elements with one valence electron (like alkali metals) tend to lose that electron to form +1 ions.
- Understanding Periodic Trends: The similarity in valence electron configurations of elements within the same group explains the observed periodic trends in properties such as ionization energy, electronegativity, and atomic radius.
- Explaining Reactivity: The stability or instability of an atom's electron configuration dictates its reactivity. Atoms strive to achieve a stable noble gas configuration, influencing their chemical behavior.
- Spectroscopy: Understanding the electron configuration is critical in interpreting atomic spectra. Electron transitions between energy levels result in the emission or absorption of light, providing information about the atom's electronic structure.
Conclusion
Abbreviated electron configurations are a powerful tool for representing the electronic structure of atoms, simplifying complex notations and highlighting crucial information about chemical behavior. By understanding the rules governing electron filling and the exceptions to those rules, we can effectively utilize abbreviated configurations to predict and explain a wide range of chemical phenomena. This concise yet informative representation is indispensable for anyone seeking a deeper understanding of atomic structure and chemical bonding. Mastering abbreviated electron configurations is a cornerstone of success in chemistry studies and related fields.
Latest Posts
Latest Posts
-
The System In The Figure Below Is In Equilibrium
Mar 27, 2025
-
Poisonous Substances Produced By Some Microorganisms Are Called
Mar 27, 2025
-
In What Cell Organelle Does Photosynthesis Occur
Mar 27, 2025
-
How Many Pi And Sigma Bonds In A Triple Bond
Mar 27, 2025
-
What Is The Difference Between A Community And Population
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is An Abbreviated Electron Configuration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
