What Is A Property Of Acids
News Leon
Mar 18, 2025 · 6 min read
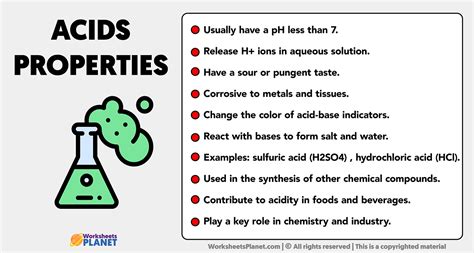
Table of Contents
- What Is A Property Of Acids
- Table of Contents
- What is a Property of Acids? A Comprehensive Guide
- Defining Acids: More Than Just a Sour Taste
- Key Properties of Acids:
- Classification of Acids: Strength and Source
- Acid Strength: Strong vs. Weak
- Acid Source: Mineral vs. Organic
- Applications of Acids: A Wide Range of Uses
- Safety Precautions When Handling Acids: Prioritize Safety
- Conclusion: Understanding Acids for a Safer and More Informed World
- Latest Posts
- Latest Posts
- Related Post
What is a Property of Acids? A Comprehensive Guide
Acids are ubiquitous in our daily lives, from the citric acid in oranges to the sulfuric acid used in car batteries. Understanding their properties is crucial in various fields, from chemistry and biology to environmental science and engineering. This comprehensive guide delves deep into the defining characteristics of acids, exploring their chemical behavior, physical properties, and practical applications.
Defining Acids: More Than Just a Sour Taste
While the sour taste of many acids is a common characteristic, it’s far from a comprehensive definition. Chemically, acids are substances that donate protons (H⁺ ions) when dissolved in water. This is the cornerstone of the Brønsted-Lowry acid-base theory, a widely accepted model that explains acid-base reactions. Another influential theory, the Lewis acid-base theory, defines acids as electron-pair acceptors. While this definition broadens the scope, the proton donation remains the most practical and commonly used definition for many purposes.
Key Properties of Acids:
Let's examine the key properties that collectively define acids:
1. Sour Taste: As mentioned earlier, many acids exhibit a characteristic sour taste. This is a sensory property, however, and should never be used as a method to identify an acid. Many strong acids are extremely corrosive and dangerous, and tasting them can lead to serious injury.
2. pH less than 7: The pH scale measures the acidity or alkalinity of a solution. A pH of 7 is neutral (like pure water). Solutions with a pH lower than 7 are acidic, with lower pH values indicating stronger acidity. The pH scale is logarithmic, meaning each whole number change represents a tenfold difference in hydrogen ion concentration.
3. Turn blue litmus paper red: Litmus paper is a common indicator used to test for acids and bases. Blue litmus paper turns red in the presence of an acid. This is a simple and convenient test, but it doesn't quantify the acidity.
4. React with bases to form salts and water: This is a fundamental characteristic of acids, known as neutralization. When an acid reacts with a base, they neutralize each other, producing a salt and water. This reaction is often exothermic, meaning it releases heat. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl, table salt) and water (H₂O):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
5. React with metals to produce hydrogen gas: Many acids, particularly strong mineral acids like hydrochloric acid and sulfuric acid, react with active metals (like zinc, magnesium, and iron) to produce hydrogen gas (H₂) and a metal salt. This is a highly exothermic reaction, often accompanied by vigorous bubbling. For example:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
6. Conduct electricity when dissolved in water: Acids, when dissolved in water, dissociate into ions, allowing them to conduct electricity. The extent of dissociation depends on the acid's strength. Strong acids completely dissociate, while weak acids only partially dissociate. The conductivity of an acid solution can be measured using a conductivity meter.
7. React with carbonates and bicarbonates to produce carbon dioxide gas: Acids react with carbonates (CO₃²⁻) and bicarbonates (HCO₃⁻) to produce carbon dioxide gas (CO₂), water, and a salt. This reaction is often used to identify the presence of carbonate or bicarbonate ions. For example:
CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + H₂O(l) + CO₂(g)
Classification of Acids: Strength and Source
Acids are classified based on their strength and source:
Acid Strength: Strong vs. Weak
Acids are categorized as either strong or weak based on their degree of ionization in water:
-
Strong acids: These acids completely dissociate into ions when dissolved in water. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), nitric acid (HNO₃), hydrobromic acid (HBr), hydroiodic acid (HI), and perchloric acid (HClO₄).
-
Weak acids: These acids only partially dissociate into ions in water. The majority of the acid remains in its molecular form. Examples include acetic acid (CH₃COOH), carbonic acid (H₂CO₃), and citric acid (C₆H₈O₇). The strength of a weak acid is represented by its acid dissociation constant (Ka).
Acid Source: Mineral vs. Organic
Acids can also be categorized based on their source:
-
Mineral acids: These are inorganic acids derived from minerals. They are generally strong acids and are often highly corrosive. Examples include sulfuric acid, nitric acid, hydrochloric acid, and phosphoric acid.
-
Organic acids: These acids are derived from organic (carbon-containing) compounds. Many organic acids are weak acids. Examples include acetic acid (found in vinegar), citric acid (found in citrus fruits), lactic acid (found in sour milk), and formic acid (found in ant stings).
Applications of Acids: A Wide Range of Uses
Acids play a crucial role in numerous industrial and everyday applications:
-
Industrial processes: Acids are widely used in chemical manufacturing, metal processing, and petroleum refining. Sulfuric acid, for instance, is a vital industrial chemical used in the production of fertilizers, detergents, and other chemicals.
-
Food and beverage industry: Acids are used as preservatives, flavor enhancers, and leavening agents. Citric acid is a common food additive, while acetic acid (vinegar) is used in various culinary applications.
-
Pharmaceuticals: Many pharmaceutical drugs contain acids or are synthesized using acid catalysts. Acids are also used in the production of various medical supplies and equipment.
-
Cleaning agents: Acids are components of many cleaning solutions, owing to their ability to dissolve certain substances. However, care must be taken as some acid cleaners can be highly corrosive.
-
Batteries: Sulfuric acid is the electrolyte in lead-acid batteries, which are commonly used in cars and other vehicles.
-
Environmental applications: Acids are sometimes used in wastewater treatment to adjust the pH of the water.
Safety Precautions When Handling Acids: Prioritize Safety
Acids can be hazardous substances, and proper safety precautions must always be taken when handling them:
-
Wear appropriate personal protective equipment (PPE): This includes safety goggles, gloves, lab coats, and potentially respirators, depending on the specific acid and concentration.
-
Handle acids carefully: Avoid spills and splashes. If a spill occurs, follow established procedures for cleanup.
-
Dilute acids carefully: Always add acid to water, never water to acid. This is because the reaction between water and acid is exothermic, and adding water to acid can cause a violent reaction and potential splashing.
-
Store acids properly: Acids should be stored in appropriate containers, in well-ventilated areas, away from incompatible substances.
-
Consult safety data sheets (SDS): Before handling any acid, always consult the SDS for detailed information on safety precautions, handling procedures, and emergency response.
Conclusion: Understanding Acids for a Safer and More Informed World
Acids are fundamental chemical substances with a wide range of properties and applications. Understanding their behavior, characteristics, and potential hazards is crucial for safe and responsible use. This detailed overview has explored the defining properties of acids, their classification, applications, and essential safety precautions. By appreciating the multifaceted nature of acids, we can harness their benefits while mitigating potential risks, contributing to a safer and more informed world.
Latest Posts
Latest Posts
-
Which Hormone Is Not Produced By The Anterior Pituitary Gland
Mar 19, 2025
-
How Many Lenses Does A Compound Microscope Have
Mar 19, 2025
-
40 19 K Protons Neutrons And Electrons
Mar 19, 2025
-
Okazaki Fragments Are Joined Together By
Mar 19, 2025
-
What Is The Relationship Between The Two Molecules Shown Below
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is A Property Of Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
