What Information Does The Electronic Configuration Of An Atom Provide
News Leon
Mar 25, 2025 · 6 min read
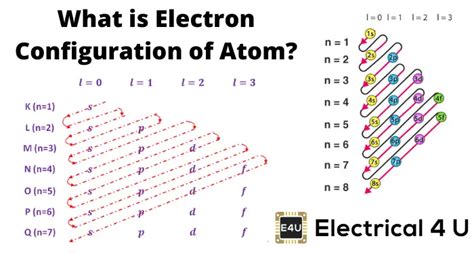
Table of Contents
What Information Does the Electronic Configuration of an Atom Provide?
The electronic configuration of an atom is a fundamental concept in chemistry and physics, providing a wealth of information about its properties and behavior. Understanding this configuration is crucial for predicting an element's reactivity, its bonding characteristics, and its position within the periodic table. This article delves deep into the details, exploring not only what the configuration tells us directly but also the indirect inferences we can make based on this crucial piece of atomic information.
Understanding Electronic Configuration: A Foundation
Before exploring the wealth of information contained within an electronic configuration, let's first establish a firm understanding of what it represents. An electronic configuration describes the arrangement of electrons within the different energy levels and sublevels of an atom. This arrangement is governed by the principles of quantum mechanics, specifically the Pauli Exclusion Principle and Hund's Rule.
The configuration is typically represented using a notation that specifies the principal energy level (n), the subshell (s, p, d, or f), and the number of electrons in each subshell. For example, the electronic configuration of carbon (atomic number 6) is 1s²2s²2p². This tells us that:
- 1s²: There are two electrons in the 1s subshell (principal energy level 1, s subshell).
- 2s²: There are two electrons in the 2s subshell (principal energy level 2, s subshell).
- 2p²: There are two electrons in the 2p subshell (principal energy level 2, p subshell).
This seemingly simple notation unlocks a surprisingly vast amount of information.
Information Directly Provided by Electronic Configuration
The electronic configuration provides direct insight into several key properties:
1. The Number of Valence Electrons: Predicting Reactivity
Perhaps the most readily apparent information is the number of valence electrons. Valence electrons are the electrons in the outermost shell (highest principal quantum number, n) of an atom. These electrons are the primary participants in chemical bonding and determine an element's reactivity.
For example, looking at the configuration of carbon (1s²2s²2p²), we see that it has four valence electrons (two in the 2s and two in the 2p subshell). This explains carbon's tetravalency – its ability to form four covalent bonds, contributing to its immense importance in organic chemistry. Elements with similar valence electron numbers often exhibit similar chemical properties, which is the foundation of the periodic table's organization. Noble gases, with their complete outer shells (typically eight electrons, the octet rule), are exceptionally unreactive because they have no tendency to gain or lose electrons to achieve stability.
2. The Identity of the Element: Atomic Number and Position in the Periodic Table
The electronic configuration directly determines the atomic number of an element. The atomic number is simply the total number of electrons (and protons) in a neutral atom. By summing the electrons from each subshell in the configuration, we obtain the atomic number, which uniquely identifies the element.
Furthermore, the electronic configuration dictates the element's position within the periodic table. Elements in the same group (vertical column) possess similar valence electron configurations, hence sharing similar chemical properties. For instance, all alkali metals (Group 1) have one valence electron (ns¹ configuration), while all halogens (Group 17) have seven (ns²np⁵ configuration). This arrangement is a direct consequence of the periodic filling of electron subshells according to the Aufbau principle.
3. Paramagnetism and Diamagnetism: Unpaired Electrons and Magnetic Properties
The electronic configuration also dictates the magnetic properties of an atom. Atoms with unpaired electrons in their orbitals are paramagnetic, meaning they are attracted to magnetic fields. Conversely, atoms with all electrons paired are diamagnetic, meaning they are slightly repelled by magnetic fields.
Consider oxygen (1s²2s²2p⁴). The 2p subshell has four electrons, resulting in two paired and two unpaired electrons. Therefore, oxygen is paramagnetic. On the other hand, magnesium (1s²2s²2p⁶3s²), with all its electrons paired, is diamagnetic. This information is crucial in various spectroscopic techniques and material science applications.
Information Indirectly Inferred from Electronic Configuration
While the direct information is readily apparent, the electronic configuration allows us to infer many other properties:
4. Ionization Energy and Electron Affinity: Energy Changes During Electron Transfer
The electronic configuration provides insights into the energy required to remove an electron (ionization energy) or the energy released upon adding an electron (electron affinity). Elements with tightly held valence electrons (like those with high nuclear charge and small atomic radii) have high ionization energies. Conversely, elements with a strong tendency to gain electrons (often those close to achieving a noble gas configuration) have high electron affinities. The electronic configuration helps us predict these trends, which are critical in understanding chemical reactions.
5. Atomic Radii and Ionic Radii: Size and Charge Effects
The electronic configuration, particularly the number of electron shells and the effective nuclear charge, influences the size of an atom (atomic radius) or an ion (ionic radius). As we move across a period (row) in the periodic table, atomic radii generally decrease due to increasing nuclear charge pulling electrons closer. Moving down a group, atomic radii increase due to the addition of new electron shells. Ionic radii are also influenced by the loss or gain of electrons during ion formation. The electronic configuration helps us understand these size trends.
6. Metallic and Non-metallic Character: Conductivity and Bonding Trends
The electronic configuration plays a pivotal role in determining an element's metallic or non-metallic character. Elements with few valence electrons tend to lose electrons easily, exhibiting metallic properties such as electrical conductivity and malleability. Conversely, elements with many valence electrons tend to gain electrons, showing non-metallic properties like poor conductivity and brittleness. The electronic configuration provides a framework for understanding these trends.
7. Oxidation States and Chemical Reactivity: Predicting Compound Formation
The electronic configuration aids in predicting the possible oxidation states (charges) an element can adopt in compounds. For example, an element with one valence electron is likely to form a +1 oxidation state by losing that electron. The possible oxidation states are linked directly to the number and arrangement of valence electrons and dictate how an element participates in chemical reactions and forms compounds. This information is crucial in predicting the stoichiometry and properties of various chemical compounds.
8. Spectroscopic Properties: Energy Level Transitions and Light Absorption/Emission
Electronic configuration is fundamental in understanding the spectroscopic behavior of atoms. Electrons can absorb energy and jump to higher energy levels (excited states). When they fall back to their ground state, they emit light of specific wavelengths. The wavelengths are directly related to the energy differences between the energy levels, which are in turn determined by the electronic configuration. This principle underpins techniques like atomic absorption spectroscopy and atomic emission spectroscopy, widely used for elemental analysis.
Conclusion: A Powerful Tool for Understanding Atomic Behavior
The electronic configuration of an atom, while a seemingly simple notation, is a powerful tool for predicting and understanding a vast array of its properties and behavior. From its basic reactivity and placement in the periodic table to its more nuanced magnetic properties, ionization energies, and spectroscopic behavior, the electronic configuration provides a foundation for comprehending the fundamental nature of matter. By mastering the interpretation of electronic configurations, one unlocks a deeper understanding of the intricate relationships between atomic structure and chemical properties, opening doors to further explorations in chemistry, physics, and material science. The information it provides is not merely descriptive; it's predictive, allowing scientists to anticipate chemical reactions, synthesize novel materials, and probe the mysteries of the atomic world.
Latest Posts
Latest Posts
-
What Is The Difference Between Co And Co
Mar 28, 2025
-
How Much Is 9 Ounces In Cups
Mar 28, 2025
-
Long Run Equilibrium In Perfect Competition Results In
Mar 28, 2025
-
A Lamp Hangs Vertically From A Cord
Mar 28, 2025
-
Calculate The Rotational Inertia Of A Meter Stick
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Information Does The Electronic Configuration Of An Atom Provide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
