What Happens To A Plant Cell In Hypertonic Solution
News Leon
Mar 30, 2025 · 6 min read
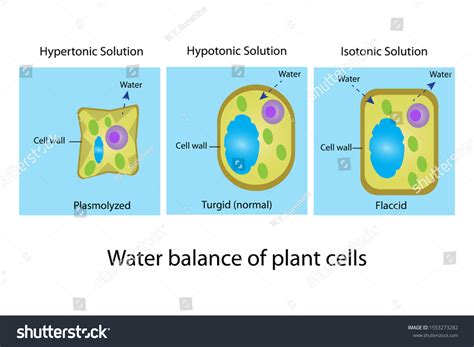
Table of Contents
What Happens to a Plant Cell in a Hypertonic Solution?
Plant cells, unlike animal cells, possess a rigid cell wall surrounding the cell membrane. This structural difference significantly impacts how they respond to changes in their external environment, particularly when exposed to hypertonic solutions. Understanding this response is crucial for comprehending plant physiology, agriculture, and even food preservation techniques. This article delves deep into the processes occurring within a plant cell when subjected to a hypertonic solution, exploring the underlying mechanisms and consequences.
Understanding Osmosis and Tonicity
Before diving into the specifics of plant cell behavior in hypertonic solutions, let's establish a firm grasp of osmosis and tonicity. Osmosis is the passive movement of water across a selectively permeable membrane from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). This movement continues until equilibrium is reached, or the water potential is equal on both sides of the membrane.
Tonicity describes the relative concentration of solutes in two solutions separated by a selectively permeable membrane. There are three main types of tonicity:
- Isotonic: The solute concentration is equal on both sides of the membrane. There's no net movement of water.
- Hypotonic: The solute concentration is lower outside the cell than inside the cell. Water moves into the cell.
- Hypertonic: The solute concentration is higher outside the cell than inside the cell. Water moves out of the cell.
It's the hypertonic environment that we'll be focusing on in relation to plant cells.
The Plant Cell's Structure: A Key Player
The structure of a plant cell plays a vital role in its response to hypertonic solutions. The key components involved are:
- Cell Wall: A rigid outer layer made primarily of cellulose. It provides structural support and protection to the cell. The cell wall's rigidity is crucial in distinguishing the plant cell's response from that of an animal cell.
- Cell Membrane (Plasma Membrane): A selectively permeable membrane that regulates the movement of substances into and out of the cell, including water.
- Vacuole: A large, central vacuole occupies a significant portion of the plant cell's volume. It stores water, nutrients, and waste products. The vacuole's turgor pressure is central to maintaining cell shape and rigidity.
- Cytoplasm: The gel-like substance filling the cell, containing various organelles.
What Happens in a Hypertonic Solution?
When a plant cell is placed in a hypertonic solution, the water potential outside the cell is lower than inside the cell. This creates a water potential gradient, driving the movement of water out of the cell via osmosis. This process unfolds in several key steps:
1. Water Loss from the Vacuole:
The initial and most significant effect is the loss of water from the central vacuole. As water exits the vacuole, its volume decreases. This directly impacts the turgor pressure, the pressure exerted by the cell contents against the cell wall. Turgor pressure is essential for maintaining the cell's shape and rigidity. Its reduction leads to a decrease in cell volume.
2. Plasmolysis:
As water continues to leave the cell, the cell membrane begins to pull away from the cell wall. This process is called plasmolysis. The cell membrane shrinks, leaving gaps between the membrane and the cell wall. The degree of plasmolysis depends on the severity of the hypertonic solution and the duration of exposure.
3. Reduced Turgor Pressure and Wilting:
The loss of turgor pressure causes the plant cell to become flaccid. If many cells within a plant undergo plasmolysis, the entire plant will wilt, as its structural support diminishes. This wilting is a visible manifestation of the water loss and the disruption of the plant's turgor pressure. Severe water loss can even lead to irreversible damage and cell death.
4. Protoplast Shrinkage:
The term protoplast refers to the entire living contents of the plant cell, excluding the cell wall. In a hypertonic solution, the protoplast shrinks, becoming smaller in volume as it loses water. This shrinkage is directly linked to the plasmolysis process.
5. Impact on Cellular Processes:
The loss of water and the subsequent changes in cell volume affect various cellular processes. Enzyme activity, metabolic reactions, and transport processes can be negatively impacted. The disruption of these processes can lead to cellular dysfunction and potential cell death if the hypertonic condition persists.
Reversal of Plasmolysis: Deplasmolysis
If the plant cell is transferred from a hypertonic solution to a hypotonic or isotonic solution, the process can be reversed. Water will move back into the cell, causing the vacuole to swell and the cell membrane to regain contact with the cell wall. This process is called deplasmolysis. The cell will regain its turgor pressure and its normal shape and function, provided the damage sustained wasn't too severe.
Practical Applications and Significance
Understanding the effects of hypertonic solutions on plant cells has significant practical applications across various fields:
-
Agriculture: Proper irrigation management is crucial to prevent water stress in plants, which leads to plasmolysis and reduced crop yields. Farmers use techniques like drip irrigation to ensure optimal water delivery to prevent hypertonic conditions in the soil.
-
Food Preservation: Osmosis plays a role in food preservation methods like pickling and salting. These processes create a hypertonic environment around microorganisms, preventing their growth and extending the shelf life of food.
-
Plant Biology Research: Studying plasmolysis provides valuable insights into plant cell physiology, water transport mechanisms, and the overall stress response of plants.
-
Medical Applications: While not directly related to plant cells, the principles of osmosis and tonicity are fundamental in understanding fluid balance in the human body and the treatment of various medical conditions.
Factors Affecting Plasmolysis
Several factors influence the extent and rate of plasmolysis:
-
Concentration of the Hypertonic Solution: A higher solute concentration in the external solution leads to a greater water potential gradient and more rapid and severe plasmolysis.
-
Type of Solute: The type of solute in the hypertonic solution can influence the rate of water movement. Some solutes may penetrate the cell membrane more readily than others, affecting the osmotic balance.
-
Temperature: Higher temperatures generally increase the rate of water movement across membranes, potentially accelerating plasmolysis.
-
Plant Species: Different plant species exhibit varying degrees of tolerance to hypertonic stress, reflecting differences in their cellular structures and physiological mechanisms.
Conclusion: A Delicate Balance
The response of a plant cell to a hypertonic solution underscores the delicate balance between water uptake and loss essential for plant survival. The rigid cell wall mitigates the potentially catastrophic effects of water loss seen in animal cells, but plasmolysis still represents a significant stress that can impair cellular function and lead to wilting and ultimately, plant death if prolonged. Understanding the intricacies of this process is crucial for developing effective strategies in agriculture, food preservation, and various research fields related to plant biology. Further research continues to explore the complex interactions between plant cells, their environment, and the cellular responses to osmotic stress, leading to innovative approaches for optimizing plant growth and enhancing our understanding of plant life.
Latest Posts
Latest Posts
-
D Dx X 1 X 2
Apr 01, 2025
-
Energy For Muscle Contraction Is Most Directly Supplied By
Apr 01, 2025
-
What Is The Charge Of A Sodium Ion
Apr 01, 2025
-
Do Lysosomes Have A Double Membrane
Apr 01, 2025
-
Which Of The Following Is True Of B Cells
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Happens To A Plant Cell In Hypertonic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
