What Does Aq Mean In A Chemical Equation
News Leon
Mar 22, 2025 · 6 min read
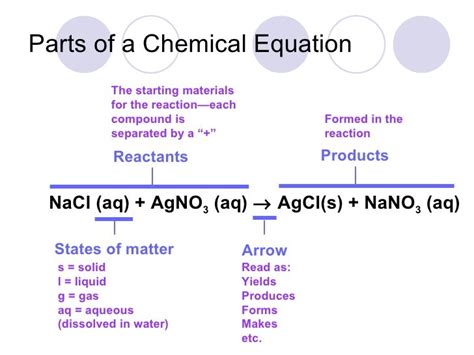
Table of Contents
What Does aq Mean in a Chemical Equation? A Comprehensive Guide
Understanding chemical equations is fundamental to grasping the principles of chemistry. These equations represent chemical reactions, showing the reactants transforming into products. Within these equations, various symbols and abbreviations are used to provide crucial information about the states of matter involved. One such abbreviation, "aq," is frequently encountered and represents a crucial aspect of the reaction. This comprehensive guide will delve deep into the meaning of "aq" in a chemical equation, exploring its significance, applications, and related concepts.
Understanding the Basics of Chemical Equations
Before we dive into the specifics of "aq," let's establish a solid foundation. A chemical equation uses chemical formulas to represent the reactants (starting materials) and products (resulting substances) in a chemical reaction. For instance, the combustion of methane can be represented as:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l)
In this equation:
- CH₄ represents methane.
- O₂ represents oxygen.
- CO₂ represents carbon dioxide.
- H₂O represents water.
The arrow (→) indicates the direction of the reaction, showing the transformation from reactants to products. The numbers in front of the formulas (coefficients) balance the equation, ensuring the same number of atoms of each element are present on both sides.
The Significance of State Symbols in Chemical Equations
Chemical equations often include state symbols in parentheses after each chemical formula. These symbols indicate the physical state of the substance at the reaction conditions, providing crucial context for understanding the reaction's nature and characteristics. Common state symbols include:
- (s): Solid
- (l): Liquid
- (g): Gas
- (aq): Aqueous
These symbols are not merely optional additions; they offer vital information about the reaction's environment, the reactants' behavior, and the reaction mechanism. For example, knowing whether a reactant is a solid, liquid, or gas influences how quickly it will react and the overall reaction rate.
Decoding "aq": Aqueous Solutions
The abbreviation "aq" stands for aqueous, meaning the substance is dissolved in water. Water is a ubiquitous solvent in chemistry, and many reactions occur in aqueous solutions. When a substance is labeled (aq), it signifies that its molecules or ions are evenly dispersed throughout the water molecules, forming a homogeneous mixture.
This is fundamentally different from the other states of matter. A solid is a structured arrangement of molecules or ions, a liquid exhibits fluidity but maintains molecular interactions, and a gas involves widely dispersed molecules with minimal interaction. An aqueous solution, however, exhibits the unique characteristics of solvation, where water molecules surround and interact with the solute molecules or ions.
Properties of Aqueous Solutions
Aqueous solutions exhibit several key properties:
- Homogeneity: The solute is evenly distributed throughout the solvent, resulting in a uniform composition.
- Electrical Conductivity: Many aqueous solutions conduct electricity, especially those containing ionic compounds that dissociate into ions in water.
- Solubility: The solubility of a substance in water determines whether it will form an aqueous solution. Polar and ionic compounds typically dissolve readily in water, while nonpolar compounds are generally insoluble.
- Reaction Medium: Water often acts as more than just a solvent; it can participate directly in the reaction mechanism, affecting the reaction rate and even the products formed.
Ionic Compounds in Aqueous Solutions: Dissociation and Ionization
The behavior of ionic compounds in aqueous solutions is particularly noteworthy. When an ionic compound dissolves in water, it undergoes dissociation, separating into its constituent ions. For instance, when sodium chloride (NaCl) dissolves in water, it dissociates into sodium ions (Na⁺) and chloride ions (Cl⁻):
NaCl(s) → Na⁺(aq) + Cl⁻(aq)
This dissociation process is facilitated by the polar nature of water molecules. The positive end of a water molecule attracts the negatively charged chloride ions, and the negative end attracts the positively charged sodium ions. This interaction weakens the ionic bonds in the crystal lattice, allowing the ions to separate and become surrounded by water molecules. This process significantly impacts the chemical properties of the solution and the reactions that can occur.
In contrast, molecular compounds may undergo ionization in aqueous solutions. This process involves the formation of ions from a neutral molecule, often through a reaction with water. A common example is the ionization of acids such as hydrochloric acid (HCl):
HCl(g) → H⁺(aq) + Cl⁻(aq)
Here, the HCl molecule reacts with water, transferring a proton (H⁺) to a water molecule, resulting in the formation of hydronium ions (H₃O⁺) and chloride ions.
The Importance of "aq" in Predicting Reaction Outcomes
The "aq" designation is crucial for understanding and predicting the outcome of chemical reactions. Knowing whether a reactant or product is in an aqueous solution helps us:
- Predict the solubility of substances: The presence of (aq) indicates that the substance is soluble in water, which is essential in designing and analyzing chemical processes.
- Understand reaction mechanisms: Aqueous solutions often provide a medium for reactions to proceed, influencing the rates and pathways of reactions.
- Determine the conductivity of solutions: The presence of ions in aqueous solutions influences their electrical conductivity, which is relevant in applications such as electrochemistry.
- Analyze the thermodynamic properties: The dissolution of a substance in water has thermodynamic implications, influencing the overall energy changes during a reaction.
Examples of Chemical Equations with "aq"
Let's consider a few examples to illustrate the use of "aq" in chemical equations:
1. Neutralization Reaction:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation represents the neutralization reaction between hydrochloric acid and sodium hydroxide, both in aqueous solutions. The products are also aqueous sodium chloride and liquid water.
2. Precipitation Reaction:
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
This reaction shows the precipitation of silver chloride (AgCl), a solid, from the reaction of aqueous silver nitrate and aqueous sodium chloride. The (aq) designations highlight that the reactants are dissolved in water, facilitating the reaction and the formation of the precipitate.
3. Redox Reaction:
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
This is a redox reaction where zinc (Zn) is oxidized, and copper ions (Cu²⁺) are reduced. The copper ions are in aqueous solution, and the resulting zinc ions are also aqueous.
Beyond "aq": Other Important Considerations
While "aq" is a crucial state symbol, it's essential to remember other factors influencing chemical reactions:
- Concentration: The concentration of the reactants significantly impacts the reaction rate and equilibrium.
- Temperature: Temperature affects the reaction rate and solubility.
- Pressure: Pressure influences gas-phase reactions and the solubility of gases in aqueous solutions.
- pH: The pH of the solution significantly affects many reactions, particularly those involving acids and bases.
- Presence of Catalysts: Catalysts can speed up reactions without being consumed themselves.
Understanding these factors alongside the state symbols provides a complete picture of the chemical process.
Conclusion
The abbreviation "aq" in a chemical equation represents an aqueous solution—a crucial aspect of many chemical reactions. Understanding the meaning and implications of "aq" is fundamental to interpreting chemical equations accurately and predicting reaction outcomes. It signifies that the substance is dissolved in water, enabling dissociation or ionization, influencing reaction mechanisms, and affecting the overall properties of the reaction mixture. By considering "aq" alongside other state symbols and reaction conditions, we gain a comprehensive understanding of the chemical process. The detailed study of aqueous solutions is key to mastering various areas of chemistry, from acid-base reactions to solubility equilibrium. Mastering the use and interpretation of "aq" is an important step in becoming proficient in chemistry.
Latest Posts
Latest Posts
-
How Far Can Light Travel In One Second
Mar 23, 2025
-
Epithelial Tissues Bottom Layer Of Cells Rests On A
Mar 23, 2025
-
In The Figure Shown What Is The Value Of X
Mar 23, 2025
-
What Is The Molar Mass Of Ccl4
Mar 23, 2025
-
Oxidation Number Of Oxygen In Ko2
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Does Aq Mean In A Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
