What Are Two Contractile Proteins Found In A Myofibril
News Leon
Mar 17, 2025 · 7 min read
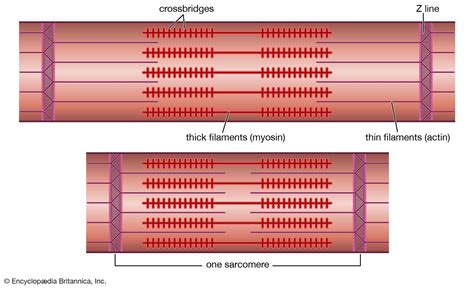
Table of Contents
What are the Two Contractile Proteins Found in a Myofibril?
The human body is a marvel of biological engineering, capable of a vast array of movements, from the delicate tap of a finger to the powerful stride of a runner. This incredible capacity for movement hinges on the intricate machinery of muscle cells, specifically the contractile proteins within their myofibrils. Understanding these proteins is key to comprehending how muscles contract and generate force. This article delves deep into the two primary contractile proteins found within myofibrils: actin and myosin. We'll explore their structures, functions, and the intricate dance they perform to power muscle contraction.
The Powerhouse Pair: Actin and Myosin
Myofibrils, the cylindrical structures within muscle fibers, are the fundamental units of muscle contraction. These highly organized bundles are composed primarily of two proteins: actin and myosin. These proteins interact in a precise and coordinated manner to generate the force necessary for movement. Let's examine each in detail.
Actin: The Thin Filament's Foundation
Actin is a globular protein (G-actin) that polymerizes to form long, fibrous filaments (F-actin). These filaments constitute the thin filaments of the myofibril, appearing as lighter bands under a microscope. Each F-actin filament is a double helix of G-actin monomers, arranged in a head-to-tail fashion.
Key features of Actin:
- Polymerization: The ability of G-actin to polymerize into F-actin is crucial for filament formation and muscle function. This process is regulated by various factors, ensuring controlled growth and disassembly of the thin filaments.
- Binding Sites: Each G-actin monomer possesses a myosin-binding site, the key location where myosin heads interact during muscle contraction. These binding sites are crucial for the cross-bridge cycle.
- Regulatory Proteins: Associated with actin are two regulatory proteins: tropomyosin and troponin. Tropomyosin wraps around the actin filament, covering the myosin-binding sites in a relaxed muscle. Troponin, a complex of three proteins, plays a crucial role in regulating the interaction between actin and myosin by controlling the position of tropomyosin. This regulatory mechanism is essential for calcium-dependent muscle contraction.
- Isoforms: Different isoforms of actin exist in various muscle types, reflecting the specialized needs of different tissues. For example, α-actin is found predominantly in skeletal and cardiac muscle, while γ-actin is more common in smooth muscle. These subtle variations in structure can influence the contractile properties of the muscle.
Myosin: The Molecular Motor
Myosin is a much larger and more complex protein than actin. It's a motor protein, meaning it converts chemical energy (ATP) into mechanical energy, driving muscle contraction. Myosin molecules assemble into thick filaments, appearing as darker bands under a microscope. Each myosin molecule has a long tail and a globular head region.
Key features of Myosin:
- Head Region: The myosin head possesses an ATP-binding site and an actin-binding site. The interaction between the myosin head and actin is the engine driving muscle contraction. The myosin head undergoes conformational changes powered by ATP hydrolysis, facilitating movement along the actin filament.
- Tail Region: The tail region of myosin is responsible for the assembly of myosin molecules into thick filaments. These filaments are bipolar, meaning they have myosin heads projecting from both ends.
- ATPase Activity: Myosin possesses intrinsic ATPase activity, meaning it can hydrolyze ATP to release energy. This energy fuels the conformational changes in the myosin head, enabling it to "walk" along the actin filament.
- Cross-Bridge Cycle: The interaction between myosin and actin, involving repeated cycles of attachment, movement, and detachment, is known as the cross-bridge cycle. This cycle is responsible for generating the force of muscle contraction. The precise steps of the cross-bridge cycle involve ATP binding, hydrolysis, phosphate release, and ADP release, each causing a conformational change in the myosin head.
- Isoforms: Similar to actin, myosin also exists in various isoforms, with different isoforms being expressed in different muscle types. These variations influence the speed and force-generating capacity of the muscle. For instance, Myosin type I is slow-twitch, while type II is fast-twitch.
The Dance of Contraction: The Sliding Filament Theory
The interaction between actin and myosin is the basis of the sliding filament theory, which explains how muscle contraction occurs. In essence, the thin filaments (actin) slide past the thick filaments (myosin), causing the sarcomeres (the basic contractile units of the myofibril) to shorten. This shortening of sarcomeres results in the overall contraction of the muscle fiber.
Key steps in the sliding filament theory:
-
Calcium Ion Release: The process begins with the release of calcium ions (Ca²⁺) from the sarcoplasmic reticulum (SR), a specialized intracellular calcium store.
-
Tropomyosin Shift: The increase in cytosolic Ca²⁺ concentration binds to troponin C, causing a conformational change in troponin and tropomyosin. This shift exposes the myosin-binding sites on the actin filament.
-
Cross-Bridge Formation: Myosin heads, now able to bind to the exposed sites on actin, form cross-bridges.
-
Power Stroke: ATP hydrolysis provides the energy for the myosin head to pivot, generating a power stroke that pulls the actin filament towards the center of the sarcomere.
-
Cross-Bridge Detachment: ATP binding to the myosin head causes it to detach from actin.
-
Myosin Head Reactivation: The myosin head then returns to its original conformation, ready to bind to another actin molecule and repeat the cycle.
-
Sarcomere Shortening: This continuous cycle of cross-bridge formation, power stroke, detachment, and reactivation causes the thin filaments to slide over the thick filaments, resulting in sarcomere shortening and muscle contraction.
-
Calcium Removal: Muscle relaxation occurs when Ca²⁺ is actively pumped back into the SR, leading to the repositioning of tropomyosin and the cessation of cross-bridge cycling.
Variations in Actin and Myosin: Muscle Fiber Types
The specific isoforms of actin and myosin expressed in a muscle fiber contribute significantly to its functional properties, determining its speed of contraction, force-generating capacity, and resistance to fatigue. Different muscle fiber types, categorized as slow-twitch (type I) and fast-twitch (type II), exhibit distinct characteristics.
-
Slow-twitch fibers (Type I): These fibers are characterized by their high myoglobin content (giving them a reddish appearance), abundant mitochondria, and slow ATPase activity. They are adapted for sustained contractions and are resistant to fatigue. They rely primarily on oxidative phosphorylation for ATP production. They utilize Myosin type I.
-
Fast-twitch fibers (Type II): These fibers have a lower myoglobin content (appearing whiter), fewer mitochondria, and fast ATPase activity. They are capable of rapid, powerful contractions but fatigue quickly. They rely more on anaerobic glycolysis for ATP production. These fibers are further subdivided into subtypes (IIA, IIX, IIB) based on their metabolic characteristics and myosin isoforms (Type IIa, Type IIx, Type IIb).
Clinical Significance: Muscle Disorders
Dysfunctions in actin and myosin can lead to various muscle disorders. Mutations in genes encoding these proteins or their associated regulatory proteins can cause a range of conditions, including:
-
Muscular Dystrophies: A group of inherited diseases characterized by progressive muscle weakness and degeneration. Mutations in dystrophin, a protein that links the cytoskeleton to the extracellular matrix, are responsible for Duchenne muscular dystrophy. While not directly related to actin and myosin, the disruption of this structural link can indirectly affect their function.
-
Myopathies: A broader term encompassing various muscle diseases, including those affecting the contractile apparatus. Some myopathies are caused by mutations in genes encoding actin or myosin isoforms, leading to impaired muscle function.
-
Congenital Myopathies: Rare muscle disorders present at birth, often involving defects in the structure or function of contractile proteins.
Conclusion: A Symphony of Protein Interaction
Actin and myosin, the two primary contractile proteins in myofibrils, engage in a precisely choreographed interaction that underpins muscle contraction. Their intricate structure, combined with the regulatory roles of tropomyosin and troponin, and the energy provided by ATP hydrolysis, allows for the remarkable capacity of muscles to generate force and movement. Understanding the structure and function of these proteins is crucial not only for comprehending the mechanics of movement but also for developing treatments for various muscle disorders. Further research into the intricacies of actin and myosin continues to unveil fascinating details about the cellular mechanisms driving the power and precision of human movement. The field is dynamic, with ongoing studies exploring the nuances of different isoforms, the roles of accessory proteins, and the complex regulatory networks controlling muscle contraction. This intricate interplay of proteins provides a robust and adaptable system that powers the movements that define our lives.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Form Of Precipitation
Mar 18, 2025
-
Which Statement About Natural Selection Is True
Mar 18, 2025
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Are Two Contractile Proteins Found In A Myofibril . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
