Water Is Referred To As The Universal Solvent Because
News Leon
Mar 23, 2025 · 6 min read
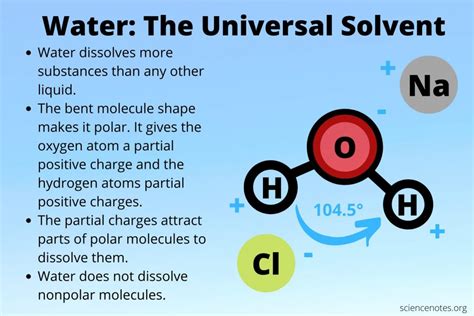
Table of Contents
- Water Is Referred To As The Universal Solvent Because
- Table of Contents
- Water: The Universal Solvent – Why It's Essential to Life and So Much More
- The Polarity of Water: The Key to its Solvency
- How Polarity Enables Dissolution
- Hydrogen Bonding: Strengthening Water's Solvent Power
- The Role of Hydrogen Bonding in Biological Systems
- Limitations of Water's Solvent Power: Not a Universal Solver After All
- The Importance of Water as a Solvent in Various Applications
- Biological Systems
- Industrial Applications
- Environmental Significance
- Conclusion: Water – The Solvent of Life and Beyond
- Latest Posts
- Latest Posts
- Related Post
Water: The Universal Solvent – Why It's Essential to Life and So Much More
Water, a seemingly simple molecule composed of two hydrogen atoms and one oxygen atom (H₂O), is far from simple in its properties and impact. Its remarkable ability to dissolve a wide range of substances earns it the title of the universal solvent. This seemingly straightforward characteristic underpins countless natural processes, industrial applications, and even the very existence of life as we know it. This article will delve into the scientific reasons behind water's solvent prowess, exploring its unique properties and the profound consequences of its dissolving power.
The Polarity of Water: The Key to its Solvency
The secret to water's exceptional solvent capabilities lies in its polarity. Unlike many other molecules, water is a polar molecule. This means that the electrons within the molecule are not evenly distributed. The oxygen atom is more electronegative than the hydrogen atoms, meaning it attracts electrons more strongly. This creates a slightly negative charge (δ-) near the oxygen atom and slightly positive charges (δ+) near the hydrogen atoms. This uneven distribution of charge creates a dipole moment, essentially making the water molecule act like a tiny magnet.
How Polarity Enables Dissolution
This polarity allows water to effectively interact with and dissolve a wide variety of substances, particularly ionic compounds and polar molecules.
-
Dissolving Ionic Compounds: Ionic compounds, like table salt (NaCl), are composed of positively charged ions (cations) and negatively charged ions (anions). The slightly negative oxygen atoms in water molecules are attracted to the positive cations, while the slightly positive hydrogen atoms are attracted to the negative anions. This attraction overcomes the electrostatic forces holding the ions together in the crystal lattice, causing the ions to separate and become surrounded by water molecules, a process called hydration. This process effectively dissolves the ionic compound.
-
Dissolving Polar Molecules: Polar molecules, like sugar (sucrose), possess regions of partial positive and negative charge due to an uneven distribution of electrons within the molecule. The slightly positive and negative regions of water molecules interact with the corresponding regions of the polar molecule, creating hydrogen bonds and facilitating dissolution. The polar molecules become surrounded by water molecules, preventing them from re-aggregating and maintaining them in solution.
Hydrogen Bonding: Strengthening Water's Solvent Power
Water's polarity also leads to the formation of hydrogen bonds. These are relatively weak bonds that form between the slightly positive hydrogen atom of one water molecule and the slightly negative oxygen atom of another. While individually weak, the collective effect of numerous hydrogen bonds is significant. These bonds contribute significantly to water's high surface tension, boiling point, and its ability to dissolve many substances. The hydrogen bonds effectively "cage" the dissolved ions or molecules, preventing them from re-forming crystals or aggregates and keeping them in solution.
The Role of Hydrogen Bonding in Biological Systems
The unique properties of water, stemming from hydrogen bonding, are crucial for life. The high heat capacity of water helps regulate temperature fluctuations in organisms, preventing drastic changes that could damage cells. The cohesive and adhesive properties of water, also a result of hydrogen bonding, are essential for transporting nutrients and waste products throughout plants and animals. The solvent properties, enabling the transport of dissolved ions and molecules, are fundamental to all biological processes.
Limitations of Water's Solvent Power: Not a Universal Solver After All
While water is an excellent solvent for many substances, it's not a universal solvent in the strictest sense. It does have limitations:
-
Nonpolar Substances: Water is a poor solvent for nonpolar substances, such as oils and fats. Nonpolar molecules lack significant regions of partial charge, so they do not interact strongly with water molecules. Instead of dissolving, nonpolar substances tend to clump together, forming separate phases from water (like oil floating on water). This is due to the hydrophobic effect, where nonpolar molecules prefer to interact with each other rather than with polar water molecules.
-
Some Ionic Compounds: While water dissolves many ionic compounds, some ionic compounds with strong ionic bonds are less soluble in water. The strength of the ionic bonds might outweigh the attractive forces between the ions and water molecules.
-
Concentration Limits: Even for substances that are highly soluble in water, there's a limit to how much can be dissolved in a given amount of water. Once this limit, the saturation point, is reached, no more solute can dissolve, and excess solute will remain undissolved.
The Importance of Water as a Solvent in Various Applications
Water's solvent properties have far-reaching implications in numerous fields:
Biological Systems
-
Cellular Processes: Water acts as the primary solvent within cells, facilitating transport of nutrients, removal of waste products, and participation in countless biochemical reactions. Enzymes, the catalysts of biological reactions, require a specific aqueous environment to function optimally.
-
Blood: Blood, the vital fluid that transports oxygen, nutrients, and hormones throughout the body, is primarily water-based. Dissolved ions, proteins, and gases are transported effectively in this aqueous medium.
-
Photosynthesis and Respiration: These crucial processes for plant and animal life both depend heavily on water's solvent properties to transport reactants and products.
Industrial Applications
-
Cleaning: Water is an essential component of many cleaning solutions, effectively dissolving dirt, grease, and other contaminants.
-
Manufacturing: Many industrial processes rely on water as a solvent or a reaction medium. It's used in the production of pharmaceuticals, plastics, and other materials.
-
Chemical Reactions: Water acts as both a solvent and a reactant in countless chemical reactions. Its polarity and ability to participate in hydrogen bonding make it a crucial component in many industrial processes.
Environmental Significance
-
Weather Patterns: Water's solvent properties are crucial for the formation of clouds and precipitation. Water vapor in the atmosphere dissolves various gases and particles, affecting weather patterns.
-
Erosion and Weathering: Water's ability to dissolve minerals contributes to erosion and weathering processes, shaping the Earth's landscape.
-
Pollution: Water's solvent capacity makes it susceptible to pollution. Many pollutants are easily dissolved in water, potentially contaminating water sources and harming ecosystems.
Conclusion: Water – The Solvent of Life and Beyond
Water's remarkable ability to act as a universal solvent is a consequence of its unique molecular structure and properties, particularly its polarity and the resulting hydrogen bonding. This seemingly simple characteristic underpins countless natural processes, from the basic functioning of cells to the intricate dynamics of weather patterns. Its importance extends to various industrial applications, highlighting its indispensable role in diverse sectors. Understanding the significance of water as the universal solvent is paramount for comprehending the complexities of life, addressing environmental concerns, and developing new technologies. While not truly a universal solvent for every substance, its unparalleled ability to dissolve a vast array of molecules firmly establishes its crucial role in our world. Further research into water’s properties will undoubtedly continue to unlock its secrets and expand our understanding of its profound influence on the planet and beyond.
Latest Posts
Latest Posts
-
Most Stable Measure Of Central Tendency
Mar 25, 2025
-
Which Of The Following Is Not A Sexually Transmitted Infection
Mar 25, 2025
-
168 Cm In Inches And Feet
Mar 25, 2025
-
Which Of The Following Has The Lowest Freezing Point
Mar 25, 2025
-
Biped Is To Quadruped Ostrich Is To
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Water Is Referred To As The Universal Solvent Because . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
