Thick Filaments Are Made Of The Protein
News Leon
Mar 24, 2025 · 6 min read
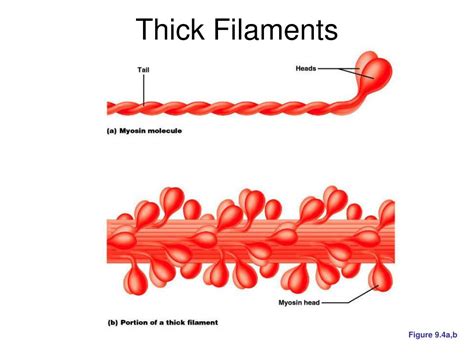
Table of Contents
Thick Filaments: A Deep Dive into Myosin and Muscle Contraction
Thick filaments, the powerhouses of muscle contraction, are predominantly composed of the protein myosin. Understanding myosin's structure and function is key to comprehending how muscles generate force and movement. This article will delve into the intricacies of thick filaments, exploring their composition, organization, and crucial role in the process of muscle contraction. We'll also touch upon the different types of myosin and their specific functions within various muscle types.
The Myosin Molecule: Structure and Function
Myosin, a motor protein, is a complex molecule with a unique structure perfectly suited for its role in generating force. Each myosin molecule is a hexamer, comprised of two heavy chains and four light chains.
The Myosin Heavy Chains: The Backbone of Power
The myosin heavy chains (MHC) are the dominant components, forming the backbone of the myosin molecule. Each heavy chain has a globular head region, also known as the S1 head, and a long, alpha-helical tail region. The S1 head possesses ATPase activity, an enzyme that hydrolyzes ATP (adenosine triphosphate) to ADP (adenosine diphosphate) and inorganic phosphate (Pi). This hydrolysis reaction is the crucial energy source driving muscle contraction.
The S1 head contains binding sites for both actin (the protein that makes up thin filaments) and ATP. The interaction between the myosin head and actin filaments is the fundamental mechanism behind muscle contraction. The precise arrangement and conformational changes of the myosin head during the ATPase cycle are vital for the generation of force.
The tail regions of the two heavy chains intertwine to form a coiled-coil structure, which contributes to the overall structural integrity of the myosin molecule and its assembly into thick filaments. Different isoforms of MHC exist, contributing to the diversity of muscle fiber types and their contractile properties.
The Myosin Light Chains: Regulators of Contraction
The myosin light chains (MLC) are smaller proteins associated with the S1 head of each heavy chain. While their exact roles are still being investigated, they are believed to play important regulatory roles in muscle contraction. They influence the interaction between the myosin head and actin, affecting the speed and efficiency of the contractile process. Different types of MLCs exist, and their specific functions and isoform variations are areas of ongoing research, influencing the fine-tuning of muscle contraction.
Thick Filament Assembly: A Precise Arrangement of Myosin Molecules
The individual myosin molecules don't operate in isolation; they assemble into highly organized structures called thick filaments. This assembly is a complex process, involving specific interactions between the myosin tails and other accessory proteins.
The Bipolar Structure: A Key Feature of Thick Filaments
Thick filaments are characterized by their bipolar structure. This means that the myosin molecules are arranged with their heads pointing outwards at both ends of the filament, while their tails are oriented towards the center. This arrangement is crucial for the simultaneous interaction with actin filaments from both directions, enabling the bidirectional force generation during muscle contraction. The bare zone in the center lacks myosin heads, contributing to the filament’s distinct structure.
Accessory Proteins: Supporting Roles in Thick Filament Formation
Several accessory proteins play supporting roles in the assembly and stabilization of thick filaments. These proteins contribute to the precise arrangement of myosin molecules, ensuring the proper bipolar structure and functionality. The roles and types of these accessory proteins can vary among muscle types.
The Sliding Filament Theory: Thick Filaments in Action
The interaction between thick and thin filaments is explained by the sliding filament theory. This theory postulates that muscle contraction occurs due to the sliding of thin filaments over thick filaments, reducing the distance between the Z-lines (structural components of the sarcomere, the basic contractile unit of muscle). The myosin heads, through their interaction with actin, generate the force necessary for this sliding movement.
The Cross-Bridge Cycle: A Repetitive Cycle of Attachment and Detachment
The interaction between myosin heads and actin filaments involves a cyclical process known as the cross-bridge cycle. This cycle consists of several steps:
-
Attachment: The myosin head, in its high-energy conformation (bound to ADP and Pi), binds to actin.
-
Power Stroke: The release of Pi triggers a conformational change in the myosin head, causing it to rotate and pull the actin filament towards the center of the sarcomere. This is the power stroke, generating the force of muscle contraction.
-
Detachment: The binding of ATP to the myosin head causes it to detach from actin.
-
Cocking: ATP hydrolysis to ADP and Pi returns the myosin head to its high-energy conformation, ready to bind to another actin molecule and repeat the cycle.
This cycle is repeated numerous times across many myosin heads, creating a coordinated movement of thin filaments along the thick filaments and resulting in muscle contraction. The regulation of this cycle through calcium ions and other regulatory proteins is vital for controlling the timing and intensity of muscle contraction.
Myosin Isoforms and Muscle Fiber Types
Different isoforms of myosin heavy chains exist, leading to variations in muscle fiber types. These isoforms affect the contractile properties of muscle fibers, such as speed of contraction, force generation, and resistance to fatigue.
Type I (Slow-Twitch) Muscle Fibers
Type I muscle fibers, also known as slow-twitch fibers, contain MHC isoforms that have slower ATPase activity. These fibers are more resistant to fatigue and are primarily involved in sustained contractions. They are rich in mitochondria, supporting their aerobic metabolism.
Type II (Fast-Twitch) Muscle Fibers
Type II muscle fibers, or fast-twitch fibers, contain MHC isoforms with faster ATPase activity. These fibers generate greater force but fatigue more quickly. They are subdivided into several subtypes with differing metabolic characteristics and contractile speeds.
Myosin Isoforms and Disease
Variations and mutations in myosin genes can lead to various muscle disorders, affecting muscle function and causing conditions like cardiomyopathies (heart muscle diseases) and muscular dystrophies. Understanding the different myosin isoforms and their roles is crucial for diagnosing and treating these conditions.
Conclusion: Thick Filaments – The Engine of Movement
Thick filaments, primarily composed of myosin, are essential components of muscle contraction. The intricate structure of myosin, its interaction with actin, and the regulated cross-bridge cycle are fundamental to generating the force that enables movement. The diversity of myosin isoforms contributes to the wide range of muscle fiber types, each with its own unique properties. Ongoing research into myosin's structure, function, and regulation continues to expand our understanding of muscle biology and its implications for human health. Further investigation into the accessory proteins and their influence on thick filament assembly and function promises to reveal more detailed insights into the complexities of muscle contraction. The future of this research holds immense potential for developing innovative therapies for muscle-related diseases and enhancing athletic performance.
Latest Posts
Latest Posts
-
Which Of The Following Is A Steroid Hormone
Mar 26, 2025
-
Osmosis Refers To The Movement Of
Mar 26, 2025
-
Digit 9 Is Always At Hundreds Place
Mar 26, 2025
-
The Rime Of Ancient Mariner Summary
Mar 26, 2025
-
Which Of The Following Cannot Be A Probability
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Thick Filaments Are Made Of The Protein . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
